Characterizing the role of enterohepatic recycling in the interactions between mycophenolate mofetil and calcineurin inhibitors in renal transplant patients by pharmacokinetic modelling
- PMID: 16120063
- PMCID: PMC1884762
- DOI: 10.1111/j.1365-2125.2005.02398.x
Characterizing the role of enterohepatic recycling in the interactions between mycophenolate mofetil and calcineurin inhibitors in renal transplant patients by pharmacokinetic modelling
Abstract
Background: Controversy remains about the interaction between mycophenolate mofetil (MMF) and the calcineurin inhibitors cyclosporin (CsA) and tacrolimus (TACR). The need to double the dose of MMF in case of CsA co-administration to achieve the same mycophenolic acid (MPA) levels as in TACR co-administration, has been attributed to either inhibition by CsA of the enterohepatic cycle, or an inhibition of glucuronidation to mycophenolate glucuronide (MPAG) by TACR. We explored these interactions clinically in 64 kidney transplant patients.
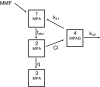
Methods: Plasma MPA/MPAG curves were determined during the first year post transplantation. Using nonlinear mixed effect modelling, MPA/MPAG data were fitted to a four-compartment model, in which a rate constant describing transfer from the fourth to the first compartment (k41), and therefore enterohepatic recycling, could be introduced.
Results: MPA and MPAG plasma concentrations were adequately described by a four-compartment model, which was significantly improved by introduction of k41 in case of TACR co-administration (minimum value of the objective function decreased by 181 points, P < 0.0001). Using this model, no statistically significant difference was observed in clearance of MPA between TACR and CsA co-administration (11.9 and 14.1 l h(-1), respectively). Total clearance of MPAG was lower in case of CsA co-administration (1.45 and 0.92 l h(-1), respectively), while there was no difference in renal clearance of MPAG (1.09 and 0.92 l h(-1), respectively).
Conclusions: Our study supplies supportive clinical evidence that inhibition of the enterohepatic cycle in case of CsA co-administration explains some of the differences observed in PK of MMF when co-administered with either TACR or CsA. This finding may have clinical consequences for the immunosuppressive management of kidney transplant patients.
Figures


References
-
- Sollinger HW. Mycophenolate mofetil for the prevention of acute rejections in primary cadaveric renal allograft recipients. US Renal transplant Mycophenolate Study Group. Transplantation. 1995;60(3):225–32. - PubMed
-
- Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Marsh JW, McCauley J, Johnston J, Randhawa P, Irish W, Gritsch HA, Naragh R, Hakala TR, Fung JJ, Starzl TE. A prospective, randomized trial of tacrolimus/prednisone versus tacrolimus/prednisone/mycophenolate mofetil in renal transplant recipients. Transplantation. 1999;67(3):411–5. - PMC - PubMed
-
- Keown PA, Landsberg D, Halloran PF, Shoker A, Rush D, Jeffery J, Russell D, Stiller C, Muirhead N, Cole E, Paul L, Zaltzman J, Loertscher R, Daloze P, Dandavino R, Boucher A, Handa P, Lawen J, Belitsky P, Parfrey P. A randomized, prospective multicenter pharmacoepidemiological study of cyclosporine microemulsion in stable renal graft recipients. Report of the Canadian Neoral Renal Transplantation Study Group. Transplantation. 1996;62(12):1744–52. - PubMed
-
- Shipkova M, Armstrong VW, Wieland E, Niedmann PD, Schuetz E, Brenner-Weiss G, Voihsel M, Braun F, Oellerich M. Identification of glucoside and carboxyl-linked glucuronide conjugates of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mophetil. Br J Clin Pharmacol. 1999;126:1075–82. - PMC - PubMed
-
- Bullingham RES, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 2003;34(6):429–55. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

