Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic
- PMID: 16120068
- PMCID: PMC1884776
- DOI: 10.1111/j.1365-2125.2005.02413.x
Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic
Abstract
Background: Therapeutic failure with antiretroviral therapy (ART) is a substantial issue where viral rebound, viral resistance and drug-related toxicity remain serious concerns. Drug exposure-response relationships have been described for the protease inhibitors, pharmacokinetic variability is substantial for this class of drugs and drug interactions can also alter ART exposure. Given this background we established a therapeutic drug monitoring (TDM) service to monitor atazanavir (ATV) plasma concentrations early after the therapy was made available to treatment-experienced people infected with HIV who were managed in a clinical setting.
Methods: This was a prospective observational study which evaluated plasma samples from 110 highly treatment-experienced people with HIV using TDM and applied pharmacokinetic analysis over a five month period.
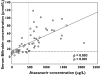
Results: ATV trough concentrations exhibited substantial intersubject variability (<25-2108 microg l(-1)). A substantial number of subjects (50%,13/26) who received ATV400 mg daily had low exposure to ATV. Serum bilirubin concentrations correlated significantly with higher ATV trough concentrations (rho = 0.803; P < 0.001) and 55% (29/53) of subjects who received ATV300/100 mg RTV daily had plasma concentrations above a proposed target concentration associated with elevated bilirubin concentrations. This study confirmed low ATV exposure in eight subjects with HIV receiving ATV 400 mg daily. Reasons for low ATV exposure in this cohort include administration of interacting drugs, including a possible interaction with ritonavir, fluticasone and ATV, impaired ATV absorption secondary to suspected achlorhydria and potential interactions with colchicine and nandrolone. Viral load remained undetectable in most of these subjects with low ATV exposure.
Conclusions: TDM and targeted pharmacokinetic studies should be viewed as fundamental tools in the development and clinical application of ART, to improve pharmacotherapy for people with HIV.
Figures

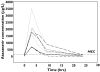
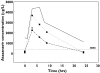
 ), 400/100 + fluticasone + cola drink (▾), 400/100 − fluticasone (▪)
), 400/100 + fluticasone + cola drink (▾), 400/100 − fluticasone (▪)References
-
- Alexander CS, Asselin JJ, Ting SL, Montaner SG, Hogg RS, Yip B, O'Shaughnessey MV, Harrigan PR. Antiretroviral concentrations in untimed plasma samples predict therapy outcome in a population with advanced disease. J Infect Dis. 2003;188:541–8. - PubMed
-
- Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, Angarano G, Colangeli V, De Luca A, Ippolito G. Insights into the reasons for discontinuation of the first highly active antriretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. AIDS. 2000;14:499–507. - PubMed
-
- Aarnoutse RE, Schapiro JM, Bouchner CA, Hekster YA, Burger DM. Therapeutic drug monitoring. An aid to optimising response to antriretroviral drugs? Drugs. 2003;63:741–53. - PubMed
-
- Goldsmith DR, Perry CM. Atazanavir. Drugs. 2003;63:1679–93. - PubMed
-
- O'Mara E, Cirincione B, Mummaneni V, Grasela T, Grasela D. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, 2001. Chicago, IL: 2001. Population pharmacodynamic (PD) assessment of the safety and antiretroviral activity of atazanavir (BMS-232632)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

