5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology
- PMID: 16120784
- PMCID: PMC6725246
- DOI: 10.1523/JNEUROSCI.1790-05.2005
5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology
Abstract
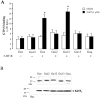
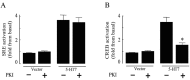
The neurotransmitter serotonin (5-HT) plays an important role in the regulation of multiple events in the CNS. We demonstrated recently a coupling between the 5-HT4 receptor and the heterotrimeric G13-protein resulting in RhoA-dependent neurite retraction and cell rounding (Ponimaskin et al., 2002). In the present study, we identified G12 as an additional G-protein that can be activated by another member of serotonin receptors, the 5-HT7 receptor. Expression of 5-HT7 receptor induced constitutive and agonist-dependent activation of a serum response element-mediated gene transcription through G12-mediated activation of small GTPases. In NIH3T3 cells, activation of the 5-HT7 receptor induced filopodia formation via a Cdc42-mediated pathway correlating with RhoA-dependent cell rounding. In mouse hippocampal neurons, activation of the endogenous 5-HT7 receptors significantly increased neurite length, whereas stimulation of 5-HT4 receptors led to a decrease in the length and number of neurites. These data demonstrate distinct roles for 5-HT7R/G12 and 5-HT4R/G13 signaling pathways in neurite outgrowth and retraction, suggesting that serotonin plays a prominent role in regulating the neuronal cytoarchitecture in addition to its classical role as neurotransmitter.
Figures
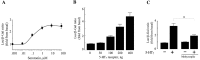
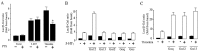

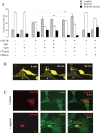
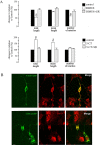
References
-
- Aktories K, Schmidt G, Just I (2000) Rho GTPases as targets of bacterial protein toxins. Biol Chem 381: 421-426. - PubMed
-
- Alves SE, Hoskin E, Lee SJ, Brake WG, Ferguson D, Luine V, Allen PB, Green-gard P, McEwen BS (2002) Serotonin mediates CA1 spine density but is not crucial for ovarian steroid regulation of synaptic plasticity in the adult rat dorsal hippocampus. Synapse 45: 143-151. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246-20249. - PubMed
-
- Azmitia EC (2001) Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull 56: 413-424. - PubMed
-
- Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083-1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
