Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans
- PMID: 16121195
- PMCID: PMC1224682
- DOI: 10.1038/sj.emboj.7600792
Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans
Abstract
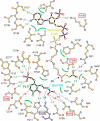
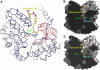
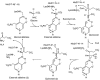
5-Aminolevulinate synthase (ALAS) is the first and rate-limiting enzyme of heme biosynthesis in humans, animals, other non-plant eukaryotes, and alpha-proteobacteria. It catalyzes the synthesis of 5-aminolevulinic acid, the first common precursor of all tetrapyrroles, from glycine and succinyl-coenzyme A (sCoA) in a pyridoxal 5'-phosphate (PLP)-dependent manner. X-linked sideroblastic anemias (XLSAs), a group of severe disorders in humans characterized by inadequate formation of heme in erythroblast mitochondria, are caused by mutations in the gene for erythroid eALAS, one of two human genes for ALAS. We present the first crystal structure of homodimeric ALAS from Rhodobacter capsulatus (ALAS(Rc)) binding its cofactor PLP. We, furthermore, present structures of ALAS(Rc) in complex with the substrates glycine or sCoA. The sequence identity of ALAS from R. capsulatus and human eALAS is 49%. XLSA-causing mutations may thus be mapped, revealing the molecular basis of XLSA in humans. Mutations are found to obstruct substrate binding, disrupt the dimer interface, or hamper the correct folding. The structure of ALAS completes the structural analysis of enzymes in heme biosynthesis.
Figures
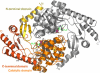


References
-
- Alexeev D, Alexeeva M, Baxter RL, Campopiano DJ, Webster SP, Sawyer L (1998) The crystal structure of 8-amino-7-oxononanoate synthase: a bacterial PLP-dependent, acyl-CoA-condensing enzyme. J Mol Biol 284: 401–419 - PubMed
-
- Andrews NC (1999) Disorders of iron metabolism. N Engl J Med 341: 1986–1995 - PubMed
-
- Bolt EL, Kryszak L, Zeilstra-Ryalls J, Shoolingin-Jordan PM, Warren MJ (1999) Characterization of the Rhodobacter sphaeroides 5-aminolaevulinic acid synthase isoenzymes, HemA and HemT, isolated from recombinant Escherichia coli. Eur J Biochem 265: 290–299 - PubMed
-
- Bottomley SS (2004) Sideroblastic anemias. In Wintrobe's Clinical Hematology, Greer J, Foerster J, Lukens JN, Rodgers GM, Paraskevas R, Glader Bertil (eds), pp 1012–1033. Philadelphia: Lippincott Williams & Wilkins
-
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54 (Part 5): 905–921 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

