Tuning genetic control through promoter engineering
- PMID: 16123130
- PMCID: PMC1200280
- DOI: 10.1073/pnas.0504604102
Tuning genetic control through promoter engineering
Erratum in
- Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):3006
Abstract
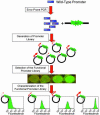
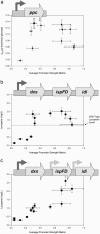
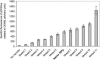
Gene function is typically evaluated by sampling the continuum of gene expression at only a few discrete points corresponding to gene knockout or overexpression. We argue that this characterization is incomplete and present a library of engineered promoters of varying strengths obtained through mutagenesis of a constitutive promoter. A multifaceted characterization of the library, especially at the single-cell level to ensure homogeneity, permitted quantitative assessment correlating the effect of gene expression levels to improved growth and product formation phenotypes in Escherichia coli. Integration of these promoters into the chromosome can allow for a quantitative accurate assessment of genetic control. To this end, we used the characterized library of promoters to assess the impact of phosphoenolpyruvate carboxylase levels on growth yield and deoxy-xylulose-P synthase levels on lycopene production. The multifaceted characterization of promoter strength enabled identification of optimal expression levels for ppc and dxs, which maximized the desired phenotype. Additionally, in a strain preengineered to produce lycopene, the response to deoxy-xylulose-P synthase levels was linear at all levels tested, indicative of a rate-limiting step, unlike the parental strain, which exhibited an optimum expression level, illustrating that optimal gene expression levels are variable and dependent on the genetic background of the strain. This promoter library concept is illustrated as being generalizable to eukaryotic organisms (Saccharomyces cerevisiae) and thus constitutes an integral platform for functional genomics, synthetic biology, and metabolic engineering endeavors.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

