Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors
- PMID: 16123383
- PMCID: PMC3381839
- DOI: 10.1634/stemcells.2005-0059
Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors
Abstract
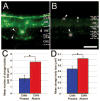
Embryonic stem (ES) cells differentiate into all cell types of the body during development, including those of the central nervous system (CNS). After transplantation, stem cells have the potential to replace host cells lost due to injury or disease or to supply host tissues with therapeutic factors and thus provide a functional benefit. In the current study, we assessed whether mouse neuralized ES cells can incorporate into retinal tissue and prevent retinal degeneration in mnd mice. These mice have an inherited lysosomal storage disease characterized by retinal and CNS degeneration. Sixteen weeks after intravitreal transplantation into adult mice, donor cells had incorporated into most layers of the retina, where they resembled retinal neurons in terms of morphology, location in the retina, and expression of cell type-specific marker proteins. Presence of these donor cells was correlated with a reduction in the sizes and numbers of lysosomal storage bodies in host retinal cells. The presence of transplanted donor cells was also accompanied by enhanced survival of host retinal neurons, particularly photoreceptors. These results demonstrate that neuralized ES cells protect host neurons from degeneration and appear to replace at least some types of lost neurons.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures




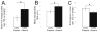
References
-
- Robertson EJ. Derivation and maintenance of embryonic stem cell cultures. Methods Mol Biol. 1997;75:173–184. - PubMed
-
- Smith AG. Embryo-derived stem cells: Of mice and men. Annu Rev Cell Dev Biol. 2001;17:435– 462. - PubMed
-
- Lumelsky N, Blondel O, Laeng P, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

