Kinetic studies on the interactions of heparin and complement proteins using surface plasmon resonance
- PMID: 16125850
- PMCID: PMC4138602
- DOI: 10.1016/j.bbagen.2005.08.003
Kinetic studies on the interactions of heparin and complement proteins using surface plasmon resonance
Abstract
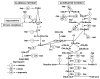
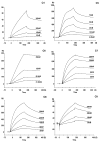
Heparin is a naturally occurring polysaccharide known to interact with complement proteins and regulate multiple steps in the complement cascade. Quantitative information, in the form of affinity constants for heparin-complement interactions, is not generally available and there are no reports of a comprehensive analysis using the same interaction method. Such information should improve our understanding of how exogenously administered pharmaceutical heparin and the related endogenous polysaccharide, heparan sulfate, regulate complement activation. The current study provides the first comprehensively analysis of the binding of various complement proteins to heparin using surface plasmon resonance (SPR). Complement proteins C1, C2, C3, C4, C5, C6, C7, C8, C9, C1INH, factor I, factor H, factor B and factor P all bind heparin but exhibit different binding kinetics and dissociation constants (Kd) ranging from 2 to 320 nM. By taking into account these Kd values and the serum concentrations of these complement proteins, the percentage of each binding to exogenously administered heparin was calculated and found to range from 2% to 41%. This study provides essential information required for the rational design of new therapeutic agents capable of regulating the complement activation.
Figures
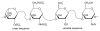


References
-
- Makrides S. Therapeutic inhibition of the complement system. Pharmacol Rev. 1998;50:59–88. - PubMed
-
- Muller-Eberhard H. Complement. Annu Rev Biochem. 1969;38:389– 414. - PubMed
-
- Muller-Eberhard H. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. - PubMed
-
- Gadjeva M, Thiel S, Jensenius JC. The mannan-binding– lectin pathway of the innate immune response. Curr Opin Immunol. 2001;13:74– 78. - PubMed
-
- Colten H, Rosen FS. Complement deficiencies. Annu Rev Immunol. 1992;10:809– 834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

