Cellular and molecular mechanisms of regulation of autoantibody production in lupus
- PMID: 16126985
- PMCID: PMC2291525
- DOI: 10.1196/annals.1361.085
Cellular and molecular mechanisms of regulation of autoantibody production in lupus
Abstract
The hyperactive interaction between helper T cells and autoimmune B cells in individuals predisposed to systemic lupus erythematosus (SLE) can be interrupted by induction of regulatory and suppressor T cells. Using two strategies-high dose tolerance to an immunoglobulin-derived peptide, and minigene vaccination with DNA encoding T cell epitopes presented by MHC class I molecules-our group has induced at least three types of regulatory/suppressive T cells. They include CD8+ T cells that suppress helper T cells by cytokine secretion, CD8+ T suppressors that kill B cells making anti-DNA antibodies, and peptide-binding CD4+CD25+ regulatory T cells that suppress B cells by direct cell contact. Each of these lymphocyte subsets suppresses anti-DNA antibody production and delays the onset of nephritis in BWF1 lupus-prone mice. Patients with SLE have amino acid sequences similar to those from murine anti-DNA antibodies used in these studies, and at similar locations in the VH regions of anti-DNA immunoglobulins. Therefore, strategies described here might ultimately be useful in therapy of the human disease.
Figures
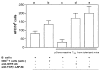
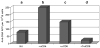
References
-
- Jiang H, Chess L. An integrated model of immunoregulation mediated by regulatory T cell subsets. Adv Immunol. 2004;83:253–288. - PubMed
-
- Kuchroo VK, Anderson AC, Waldner H, et al. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning and regulating the autopathogenic T cell repertoire. Annu Rev Immunol. 2002;20:101–123. - PubMed
-
- Scotto L, Naiyer AJ, Galluzzo S, et al. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28− suppressor cells. Hum Immunol. 2004;65:1297–1306. - PubMed
-
- Connolly DJ, Cotterill LA, Hederer RA, et al. A cDNA clone encoding the mouse Qa-1a histocompatibility antigen and proposed structure of the putative peptide binding site. J Immunol. 1993;151:6089–6098. - PubMed
-
- Jiang H, Chess L. The specific regulation of immune responses by CD8+ T cells restricted by the MHC class Ib molecule QA-1. Annu Rev Immunol. 2000;18:185–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

