Absolute bioavailability and pharmacokinetics of linezolid in hospitalized patients given enteral feedings
- PMID: 16127039
- PMCID: PMC1195442
- DOI: 10.1128/AAC.49.9.3676-3681.2005
Absolute bioavailability and pharmacokinetics of linezolid in hospitalized patients given enteral feedings
Abstract
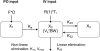
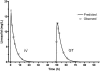
Linezolid is a new antimicrobial agent effective against drug-resistant gram-positive pathogens which are common causes of infections in hospitalized patients. Many such patients rely on the intravenous or enteral route for nutrition and drug administration. Therefore, the bioavailability of linezolid administered enterally in the presence of enteral feedings in hospitalized patients was examined. Eighteen subjects were assessed in a randomized single-dose crossover study; 12 received continuous enteral feedings, while 6 did not (controls). Both groups received linezolid 600 mg intravenously and orally (control) or enterally, with the alternate route of administration separated by a 24-h washout period. Pharmacokinetic parameters derived from noncompartmental and compartmental analysis incorporating linear and nonlinear elimination pathways were compared between groups: F, Ka, Vs, K23, K32, Vmax, Km, and K20 (bioavailability, absorption rate constant, volume of central compartment normalized to body weight, intercompartmental rate constants, maximum velocity, Michaelis-Menten constant, and elimination rate constant, respectively). Pharmacokinetic (PK) data were available from 17 patients. The linezolid oral suspension was rapidly and completely absorbed by either the oral or enteral route of administration. Bioavailability was unaltered in the presence of enteral feedings. PK estimates remain similar regardless of the model applied. At the therapeutic dose used, only slight nonlinearity in elimination was observed. A linezolid oral suspension may be administered via the enteral route to hospitalized patients without compromise in its excellent bioavailability and rapid rate of absorption. Compartmental pharmacokinetic analysis offers a more flexible study application, since bioavailability (F) can be estimated directly with intermixed intravenous/oral doses without a need for a washout period.
Figures

References
-
- Bass, J., M. V. Miles, M. B. Tennison, B. J. Holcombe, and M. D. Thorn. 1989. Effects of enteral tube feeding on the absorption and pharmacokinetic profile of carbamazepine suspension. Epilepsia 30:364-369. - PubMed
-
- Borner, K., E. Borner, and H. Lode. 2001. Determination of linezolid in human serum and urine by high-performance liquid chromatography. International J. Antimicrob. Agents 18:253-258. - PubMed
-
- Jelliffe, R., and S. Jelliffe. 1972. A computer program for estimation of creatinine clearance from unstable serum creatinine levels, age, sex and weight. Math. Biosci. 14:17-24.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

