Activin controls skin morphogenesis and wound repair predominantly via stromal cells and in a concentration-dependent manner via keratinocytes
- PMID: 16127153
- PMCID: PMC1698729
- DOI: 10.1016/S0002-9440(10)62047-0
Activin controls skin morphogenesis and wound repair predominantly via stromal cells and in a concentration-dependent manner via keratinocytes
Abstract
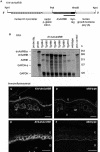
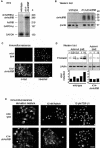
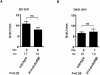
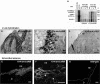
The transforming growth factor-beta family member activin is a potent regulator of skin morphogenesis and repair. Transgenic mice overexpressing activin in keratinocytes display epidermal hyper-thickening and dermal fibrosis in normal skin and enhanced granulation tissue formation after wounding. Mice overexpressing the secreted activin antagonist follistatin, however, have the opposite wound-healing phenotype. To determine whether activin affects skin morphogenesis and repair via activation of keratinocytes and/or stromal cells, we generated transgenic mice expressing a dominant-negative activin receptor IB mutant (dnActRIB) in keratinocytes. The architecture of adult skin was unaltered in these mice, but delays were observed in postnatal pelage hair follicle morphogenesis and in the first catagen-telogen transformation of hair follicles. Although dnActRIB-transgenic mice showed slightly delayed wound re-epithelialization after skin injury, the strong inhibition of granulation tissue formation seen in follistatin-transgenic mice was not observed. Therefore, although endogenous activin appeared to affect skin morphogenesis and repair predominantly via stromal cells, overexpressed activin strongly affected the epidermis. The epidermal phenotype of activin-overexpressing mice was partially rescued by breeding these animals with dnActRIB-transgenic mice. These results demonstrate that activin affects both stromal cells and keratinocytes in normal and wounded skin and that the effect on keratinocytes is dose-dependent in vivo.
Figures


References
-
- Lin SY, Morrison JR, Phillips DJ, de Kretser DM. Regulation of ovarian function by the TGF-beta superfamily and follistatin. Reproduction. 2003;126:133–148. - PubMed
-
- Kerszberg M, Wolpert L. Mechanisms for positional signalling by morphogen transport: a theoretical study. J Theor Biol. 1998;191:103–114. - PubMed
-
- Dyson S, Gurdon JB. The interpretation of position in a morphogen gradient as revealed by occupancy of activin receptors. Cell. 1998;93:557–568. - PubMed
-
- Phillips DJ. Regulation of activin’s access to the cell: why is mother nature such a control freak? Bioessays. 2000;22:689–696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

