Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice
- PMID: 16127462
- PMCID: PMC1190371
- DOI: 10.1172/JCI24819
Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice
Abstract
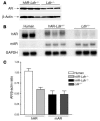
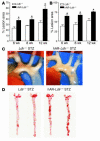
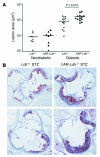
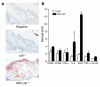
Direct evidence that hyperglycemia, rather than concomitant increases in known risk factors, induces atherosclerosis is lacking. Most diabetic mice do not exhibit a higher degree of atherosclerosis unless the development of diabetes is associated with more severe hyperlipidemia. We hypothesized that normal mice were deficient in a gene that accelerated atherosclerosis with diabetes. The gene encoding aldose reductase (AR), an enzyme that mediates the generation of toxic products from glucose, is expressed at low levels in murine compared with human tissues. Mice in which diabetes was induced through streptozotocin (STZ) treatment, but not nondiabetic mice, expressing human AR (hAR) crossed with LDL receptor-deficient (Ldlr-/-) C57BL/6 male mice had increased aortic atherosclerosis. Diabetic hAR-expressing heterozygous LDL receptor-knockout mice (Ldlr+/-) fed a cholesterol/cholic acid-containing diet also had increased aortic lesion size. Lesion area at the aortic root was increased by STZ treatment alone but was further increased by hAR expression. Macrophages from hAR-transgenic mice expressed more scavenger receptors and had greater accumulation of modified lipoproteins than macrophages from nontransgenic mice. Expression of genes that regulate regeneration of glutathione was reduced in the hAR-expressing aortas. Thus, hAR increases atherosclerosis in diabetic mice. Inhibitors of AR or other enzymes that mediate glucose toxicity could be useful in the treatment of diabetic atherosclerosis.
Figures



References
-
- Anonymous Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. - PubMed
-
- Diabetes ControlComplications Trial. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am. J. Cardiol. 1995;75:894–903. - PubMed
-
- Mathe D. Dyslipidemia and diabetes: animal models [review] Diabete Metab. 1995;21:106–111. - PubMed
-
- Keren P, et al. Effect of hyperglycemia and hyperlipidemia on atherosclerosis in LDL receptor-deficient mice: establishment of a combined model and association with heat shock protein 65 immunity. Diabetes. 2000;49:1064–1069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

