Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening
- PMID: 16127463
- PMCID: PMC1190372
- DOI: 10.1172/JCI24898
Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening
Abstract
The most common cause of cystic fibrosis (CF) is deletion of phenylalanine 508 (DeltaF508) in the CF transmembrane conductance regulator (CFTR) chloride channel. The DeltaF508 mutation produces defects in folding, stability, and channel gating. To identify small-molecule correctors of defective cellular processing, we assayed iodide flux in DeltaF508-CFTR-transfected epithelial cells using a fluorescent halide indicator. Screening of 150,000 chemically diverse compounds and more than 1,500 analogs of active compounds yielded several classes of DeltaF508-CFTR correctors (aminoarylthiazoles, quinazolinylaminopyrimidinones, and bisaminomethylbithiazoles) with micromolar potency that produced greater apical membrane chloride current than did low-temperature rescue. Correction was seen within 3-6 hours and persisted for more than 12 hours after washout. Functional correction was correlated with plasma membrane expression of complex-glycosylated DeltaF508-CFTR protein. Biochemical studies suggested a mechanism of action involving improved DeltaF508-CFTR folding at the ER and stability at the cell surface. The bisaminomethylbithiazoles corrected DeltaF508-CFTR in DeltaF508/DeltaF508 human bronchial epithelia but did not correct a different temperature-sensitive CFTR mutant (P574H-CFTR) or a dopamine receptor mutant. Small-molecule correctors may be useful in the treatment of CF caused by the DeltaF508 mutation.
Figures



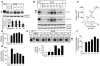


References
-
- Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations — correlation with incidence data and application to screening [review] Hum. Mutat. 2002;19:575–606. - PubMed
-
- Pilewski JM, Frizzell RA Role of CFTR in airway disease. Physiol. Rev. 1999;79:S215–S255. - PubMed
-
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol. Rev. 1999;79:S23–S45. - PubMed
-
- Denning GM, et al. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- GP0296Y01/TI_/Telethon/Italy
- DK35124/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- HL59198/HL/NHLBI NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- R01 HL059198/HL/NHLBI NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

