Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2
- PMID: 16127465
- PMCID: PMC1190374
- DOI: 10.1172/JCI25118
Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2
Abstract
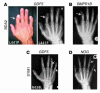
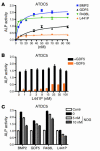
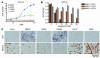
Here we describe 2 mutations in growth and differentiation factor 5 (GDF5) that alter receptor-binding affinities. They cause brachydactyly type A2 (L441P) and symphalangism (R438L), conditions previously associated with mutations in the GDF5 receptor bone morphogenetic protein receptor type 1b (BMPR1B) and the BMP antagonist NOGGIN, respectively. We expressed the mutant proteins in limb bud micromass culture and treated ATDC5 and C2C12 cells with recombinant GDF5. Our results indicated that the L441P mutant is almost inactive. The R438L mutant, in contrast, showed increased biological activity when compared with WT GDF5. Biosensor interaction analyses revealed loss of binding to BMPR1A and BMPR1B ectodomains for the L441P mutant, whereas the R438L mutant showed normal binding to BMPR1B but increased binding to BMPR1A, the receptor normally activated by BMP2. The binding to NOGGIN was normal for both mutants. Thus, the brachydactyly type A2 phenotype (L441P) is caused by inhibition of the ligand-receptor interaction, whereas the symphalangism phenotype (R438L) is caused by a loss of receptor-binding specificity, resulting in a gain of function by the acquisition of BMP2-like properties. The presented experiments have identified some of the main determinants of GDF5 receptor-binding specificity in vivo and open new prospects for generating antagonists and superagonists of GDF5.
Figures




References
-
- Sebald W, Nickel J, Zhang JL, Mueller TD. Molecular recognition in bone morphogenetic protein (BMP)/receptor interaction. Biol. Chem. 2004;385:697–710. - PubMed
-
- Luyten FP. Cartilage-derived morphogenetic protein-1. Int. J. Biochem. Cell Biol. 1997;29:1241–1244. - PubMed
-
- Nishitoh H, et al. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J. Biol. Chem. 1996;271:21345–21352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

