Contributions of cortical subventricular zone to the development of the human cerebral cortex
- PMID: 16127688
- PMCID: PMC2628573
- DOI: 10.1002/cne.20714
Contributions of cortical subventricular zone to the development of the human cerebral cortex
Abstract
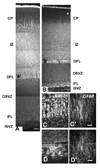
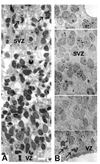
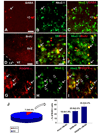
The cortical subventricular zone (SVZ), a proliferative compartment in the forebrain, has a uniquely important role during the second half of intrauterine development in human. This is best observed in numerous neonatal pathologies that result from prenatal SVZ damage. These conditions highlight a need to better understand the contribution of the SVZ to the development of the human cerebral cortex. With this goal in mind, we analyzed histological organization, cell proliferation, and molecular diversity in the human fetal SVZ from 7-27 gestational weeks (gw) using light and electron microscopy, immunohistochemistry, and in vitro methods. Complex histological organization distinguishes human cortical SVZ from that of other mammals. In vitro quantification showed that approximately 50% of cells in the VZ/SVZ region are neurons, 30% are astroglia, 15% are nestin+ cells, with other cell types representing smaller fractions. Immunolabeling with BrdU showed that a considerable number of cells ( approximately 10%) are generated in the human cortical SVZ during midgestation (18-24 gw) under in vitro conditions. Immunofluorescence with cell type-specific markers and BrdU revealed that all major cell types, neural precursors (nestin+), astroglia including radial glia (GFAP+, vimentin+), and oligodendrocyte progenitors (PDGFR-alpha+) were proliferating. An increase in the ratio of the size of the SVZ to VZ, protracted period of cell proliferation, as well as cellular and histological complexity of the human fetal SVZ are directly related to the evolutionary expansion of the human cerebral cortex.
(c) 2005 Wiley-Liss, Inc.
Figures





References
-
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Anderson SA, Martin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migration from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
-
- Andjelkovic A, Nikolic B, Pachter J, Zecevic N. Microglia/macrophages-like cells in human central nervous system during development: An immunocytochemical study. Brain Res. 1998;814:13–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

