Trans-10,cis-12, not cis-9,trans-11, conjugated linoleic acid decreases ErbB3 expression in HT-29 human colon cancer cells
- PMID: 16127743
- PMCID: PMC4320386
- DOI: 10.3748/wjg.v11.i33.5142
Trans-10,cis-12, not cis-9,trans-11, conjugated linoleic acid decreases ErbB3 expression in HT-29 human colon cancer cells
Abstract
Aim: To examine whether trans-10,cis-12 CLA (t10c12) or cis-9,trans-11 CLA (c9t11) inhibits heregulin (HRG)-beta-stimulated cell growth and HRG-beta-ErbB3 signaling in HT-29 cells.
Methods: We cultured HT-29 cells in the absence or presence of the CLA isomers and/or the ErbB3 ligand HRG-beta. MTT assay, [3H]thymidine incorporation, Annexin V staining, RT-PCR, Western blotting, immunoprecipitation, and in vitro kinase assay were performed.
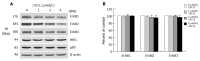
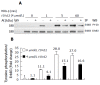
Results: HRG-beta increased cell growth, but did not prevent t10c12-induced growth inhibition. T10c12 inhibited DNA synthesis and induced apoptosis of HT-29 cells, whereas c9t11 had no effect. T10c12 decreased the levels of ErbB1, ErbB2, and ErbB3 proteins and transcripts in a dose-dependent manner, whereas c9t11 had no effect. Immunoprecipitation/Western blot studies revealed that t10c12 inhibited HRG-beta-stimulated phosphorylation of ErbB3, recruitment of the p85 subunit of phosphoinositide 3-kinase (PI3K) to ErbB3, ErbB3-associated PI3K activities, and phosphorylation of Akt. However, c9t11 had no effect on phospho Akt levels. Neither t10c12 nor c9t11 had any effect on HRG-beta-induced phosphorylation of ERK-1/2.
Conclusion: These results indicate that the inhibition of HT-29 cell growth by t10c12 may be induced via its modulation of ErbB3 signaling leading to inhibition of Akt activation.
Figures

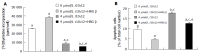


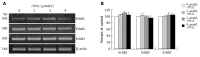




References
-
- Belury MA. Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J Nutr. 2002;132:2995–2998. - PubMed
-
- Park HS, Ryu JH, Ha YL, Park JH. Dietary conjugated linoleic acid (CLA) induces apoptosis of colonic mucosa in 1,2-dimethylhydrazine-treated rats: a possible mechanism of the anticarcinogenic effect by CLA. Br J Nutr. 2001;86:549–555. - PubMed
-
- Park HS, Cho HY, Ha YL, Park JH. Dietary conjugated linoleic acid increases the mRNA ratio of Bax/Bcl-2 in the colonic mucosa of rats. J Nutr Biochem. 2004;15:229–235. - PubMed
-
- O'Shea M, Stanton C, Devery R. Antioxidant enzyme defence responses of human MCF-7 and SW480 cancer cells to conjugated linoleic acid. Anticancer Res. 1999;19:1953–1959. - PubMed
-
- Palombo JD, Ganguly A, Bistrian BR, Menard MP. The antiproliferative effects of biologically active isomers of conjugated linoleic acid on human colorectal and prostatic cancer cells. Cancer Lett. 2002;177:163–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

