Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection
- PMID: 16129706
- PMCID: PMC2212878
- DOI: 10.1084/jem.20050821
Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection
Abstract
T cell expansion and memory formation are generally more effective when elicited by live organisms than by inactivated vaccines. Elucidation of the underlying mechanisms is important for vaccination and therapeutic strategies. We show that the massive expansion of antigen-specific CD8 T cells that occurs in response to viral infection is critically dependent on the direct action of type I interferons (IFN-Is) on CD8 T cells. By examining the response to infection with lymphocytic choriomeningitis virus using IFN-I receptor-deficient (IFN-IR(0)) and -sufficient CD8 T cells adoptively transferred into normal IFN-IR wild-type hosts, we show that the lack of direct CD8 T cell contact with IFN-I causes >99% reduction in their capacity to expand and generate memory cells. The diminished expansion of IFN-IR(0) CD8 T cells was not caused by a defect in proliferation but by poor survival during the antigen-driven proliferation phase. Thus, IFN-IR signaling in CD8 T cells is critical for the generation of effector and memory cells in response to viral infection.
Figures


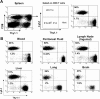
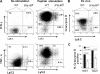
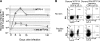





References
-
- Murali-Krishna, K., J.D. Altman, M. Suresh, D.J. Sourdive, A.J. Zajac, J. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 8:177–187. - PubMed
-
- Kaech, S.M., E.J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262. - PubMed
-
- Hochrein, H., K. Shortman, D. Vremec, B. Scott, P. Hertzog, and M. O'Keeffe. 2001. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J. Immunol. 166:5448–5456. - PubMed
-
- Stark, G.R., I.M. Kerr, B.R. Williams, R.H. Silverman, and R.D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

