Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction
- PMID: 16129797
- PMCID: PMC1456731
- DOI: 10.1161/CIRCULATIONAHA.105.537480
Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction
Abstract
Background: Recent results from animal studies suggest that stem cells may be able to home to sites of myocardial injury to assist in tissue regeneration. However, the histological interpretation of postmortem tissue, on which many of these studies are based, has recently been widely debated.
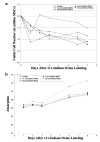
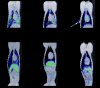
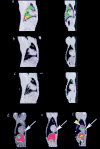
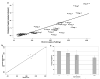
Methods and results: With the use of the high sensitivity of a combined single-photon emission CT (SPECT)/CT scanner, the in vivo trafficking of allogeneic mesenchymal stem cells (MSCs) colabeled with a radiotracer and MR contrast agent to acute myocardial infarction was dynamically determined. Redistribution of the labeled MSCs after intravenous injection from initial localization in the lungs to nontarget organs such as the liver, kidney, and spleen was observed within 24 to 48 hours after injection. Focal and diffuse uptake of MSCs in the infarcted myocardium was already visible in SPECT/CT images in the first 24 hours after injection and persisted until 7 days after injection and was validated by tissue counts of radioactivity. In contrast, MRI was unable to demonstrate targeted cardiac localization of MSCs in part because of the lower sensitivity of MRI.
Conclusions: Noninvasive radionuclide imaging is well suited to dynamically track the biodistribution and trafficking of mesenchymal stem cells to both target and nontarget organs.
Figures



References
-
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. - PubMed
-
- Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, Freilinger M, Hoger H, Elbe-Burger A, Wachtler F. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 1999;199:391–396. - PubMed
-
- Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM, Teupe C, Britten M, Dobert N, Grunwald F, Aicher A, Urbich C, Hoelzer D. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. - PubMed
-
- Bittira B, Shum-Tim D, Al-Khaldi A, Chiu RC. Mobilization and homing of bone marrow stromal cells in myocardial infarction. Eur J Cardiothorac Surg. 2003;24:393–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

