A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones
- PMID: 16129837
- PMCID: PMC1200314
- DOI: 10.1073/pnas.0506420102
A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones
Abstract
Odorants and pheromones as well as sweet- and bitter-tasting small molecules are perceived through activation of G protein-coupled chemosensory receptors. In contrast, gustatory detection of salty and sour tastes may involve direct gating of sodium channels of the DEG/ENaC family by sodium and hydrogen ions, respectively. We have found that ppk25, a Drosophila melanogaster gene encoding a DEG/ENaC channel subunit, is expressed at highest levels in the male appendages responsible for gustatory and olfactory detection of female pheromones: the legs, wings, and antennae. Mutations in the ppk25 gene reduce or even abolish male courtship response to females in the dark, conditions under which detection of female pheromones is an essential courtship-activating sensory input. In contrast, the same mutations have no effect on other behaviors tested. Importantly, ppk25 mutant males that show no response to females in the dark execute all of the normal steps of courtship behavior in the presence of visible light, suggesting that ppk25 is required for activation of courtship behavior by chemosensory perception of female pheromones. Finally, a ppk25 mutant allele predicted to encode a truncated protein has dominant-negative properties, suggesting that the normal Ppk25 protein acts as part of a multiprotein complex. Together, these results indicate that ppk25 is necessary for response to female pheromones by D. melanogaster males, and suggest that members of the DEG/ENaC family of genes play a wider role in chemical senses than previously suspected.
Figures
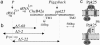

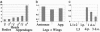
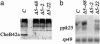
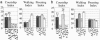

References
-
- Hall, J. C. (1994) Science 264, 1702-1714. - PubMed
-
- Greenspan, R. J. & Ferveur, J. F. (2000) Annu. Rev. Genet. 34, 205-232. - PubMed
-
- Amrein, H. (2004) Curr. Opin. Neurobiol. 14, 435-442. - PubMed
-
- Mombaerts, P. (2004) Nat. Rev. Neurosci. 5, 263-278. - PubMed
-
- Bray, S. & Amrein, H. (2003) Neuron 39, 1019-1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

