Specificity versus stability in computational protein design
- PMID: 16129838
- PMCID: PMC1200299
- DOI: 10.1073/pnas.0506124102
Specificity versus stability in computational protein design
Abstract
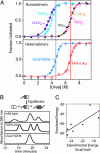
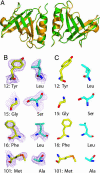
Protein-protein interactions can be designed computationally by using positive strategies that maximize the stability of the desired structure and/or by negative strategies that seek to destabilize competing states. Here, we compare the efficacy of these methods in reengineering a protein homodimer into a heterodimer. The stability-design protein (positive design only) was experimentally more stable than the specificity-design heterodimer (positive and negative design). By contrast, only the specificity-design protein assembled as a homogenous heterodimer in solution, whereas the stability-design protein formed a mixture of homodimer and heterodimer species. The experimental stabilities of the engineered proteins correlated roughly with their calculated stabilities, and the crystal structure of the specificity-design heterodimer showed most of the predicted side-chain packing interactions and a main-chain conformation indistinguishable from the wild-type structure. These results indicate that the design simulations capture important features of both stability and structure and demonstrate that negative design can be critical for attaining specificity when competing states are close in structure space.
Figures

References
-
- Dahiyat, B. I. & Mayo, S. L. (1997) Science 278, 82–87. - PubMed
-
- Looger, L. L., Dwyer, M. A., Smith, J. J. & Hellinga, H. W. (2003) Nature 423, 185–190. - PubMed
-
- Kuhlman, B., Dantas, G., Ireton, G. C., Varani, G., Stoddard, B. L. & Baker, D. (2003) Science 302, 1364–1368. - PubMed
-
- Wilson, C., Mace, J. E. & Agard, D. A. (1991) J. Mol. Biol. 220, 495–506. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

