Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: a cluster randomized trial
- PMID: 16129870
- PMCID: PMC1188186
- DOI: 10.1503/cmaj.050091
Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: a cluster randomized trial
Abstract
Background: Too few patients with nonvalvular atrial fibrillation (NVAF) receive appropriate antithrombotic therapy. We tested the short-term (primary outcome) and long-term (secondary outcome) effect of a patient decision aid on the appropriateness of antithrombotic therapy among patients with NVAF.
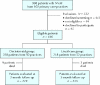
Methods: We conducted a cluster randomized trial with blinded outcome assessment involving 434 NVAF patients from 102 community-based primary care practices. Patients in the intervention group received a self-administered booklet and audiotape decision aid tailored to their personal stroke risk profile. Patients in the control group received usual care. The primary outcome measure was change in antithrombotic therapy at 3 months. Appropriateness of therapy was defined using the American College of Chest Physicians (ACCP) recommendations.
Results: The mean patient age was 72 years, and the median duration of NVAF was 5 years. In the control group, there was a 3% decrease over 3 months in the number of patients receiving therapy appropriate to their risk of stroke (40% [85/215] at baseline v. 37% [79/215] at 3 months). In the intervention group, the number of patients receiving therapy appropriate to their stroke risk increased by 9% (32% [69/219] at baseline v. 41% [89/219] at 3 months). Although the proportion of patients whose therapy met the ACCP treatment recommendations did not differ between study arms at baseline (p = 0.11) or 3 months (p = 0.44), there was a 12% absolute improvement in the number of patients receiving appropriate care in the intervention group compared with the control group at 3 months (p = 0.03). The beneficial effect of the decision aid did not persist (p = 0.44 for differences between study arms after 12 months).
Interpretation: There was short-term improvement in the appropriateness of antithrombotic care among patients with NVAF who were exposed to a decision aid, but the improvement did not persist.
Figures
References
-
- Albers GW, Dalen JE, Laupacis A, Manning WJ, Petersen P, Singer DE. Antithrombotic therapy in atrial fibrillation. Chest 2001;119(Suppl):194S-206S. - PubMed
-
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999;131:492-501. - PubMed
-
- Van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: An individual patient meta-analysis. JAMA 2002;288:2441-8. - PubMed
-
- Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation. How well do randomized trials translate into clinical practice? JAMA 2003;290:2685-92. - PubMed
-
- Bungard TJ, Ghali WA, Teo K, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med 2000;160:41-6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous