Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway
- PMID: 16131544
- PMCID: PMC1201609
- DOI: 10.1073/pnas.0506172102
Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway
Abstract
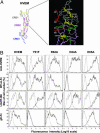
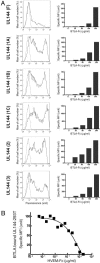
The herpesvirus entry mediator (HVEM), a member of the TNF receptor (TNFR) superfamily, can act as a molecular switch that modulates T cell activation by propagating positive signals from the TNF-related ligand LIGHT (TNFR superfamily 14), or inhibitory signals through the Ig superfamily member B and T lymphocyte attenuator (BTLA). Competitive binding analysis and mutagenesis reveals a unique BTLA binding site centered on a critical lysine residue in cysteine-rich domain 1 of HVEM. The BTLA binding site on HVEM overlaps with the binding site for the herpes simplex virus 1 envelope glycoprotein D, but is distinct from where LIGHT binds, yet glycoprotein D inhibits the binding of both ligands, potentially nullifying the pathway. The binding site on HVEM for BTLA is conserved in the orphan TNFR, UL144, present in human CMV. UL144 binds BTLA, but not LIGHT, and inhibits T cell proliferation, selectively mimicking the inhibitory cosignaling function of HVEM. The demonstration that distinct herpesviruses target the HVEM-BTLA cosignaling pathway suggests the importance of this pathway in regulating T cell activation during host defenses.
Figures


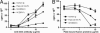
References
-
- Egen, J. G., Kuhns, M. S. & Allison, J. P. (2002) Nat. Immunol. 3, 611-618. - PubMed
-
- Sharpe, A. H. & Freeman, G. J. (2002) Nat. Rev. Immunol. 2, 116-126. - PubMed
-
- Greenwald, R. J., Latchman, Y. E. & Sharpe, A. H. (2002) Curr. Opin. Immunol. 14, 391-396. - PubMed
-
- Watanabe, N., Gavrieli, M., Sedy, J. R., Yang, J., Fallarino, F., Loftin, S. K., Hurchla, M. A., Zimmerman, N., Sim, J., Zang, X., et al. (2003) Nat. Immunol. 4, 670-679. - PubMed
-
- Han, P., Goularte, O. D., Rufner, K., Wilkinson, B. & Kaye, J. (2004) J. Immunol. 172, 5931-5939. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

