MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide
- PMID: 16134968
- PMCID: PMC1383665
- DOI: 10.1042/BJ20050959
MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide
Abstract
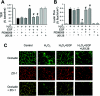
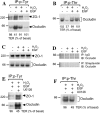
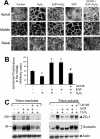
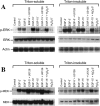
The MAPK (mitogen-activated protein kinase) pathway is a major intracellular signalling pathway involved in EGF (epithelial growth factor) receptor-mediated cell growth and differentiation. A novel function of MAPK activity in the mechanism of EGF-mediated protection of TJs (tight junctions) from H2O2 was examined in Caco-2 cell monolayers. EGF-mediated prevention of H2O2-induced increase in paracellular permeability was associated with the prevention of H2O2-induced Tyr-phosphorylation, Thr-dephosphorylation and cellular redistribution of occludin and ZO-1 (zonula occludin-1). EGF also prevented H2O2-induced disruption of the actin cytoskeleton and the dissociation of occludin and ZO-1 from the actin-rich detergent-insoluble fractions. MEK (MAPK/ERK kinase, where ERK stands for extracellular signal related kinase) inhibitors, PD98059 and U0126, completely blocked these protective effects of EGF on TJs. EGF rapidly increased the levels of phosphorylated MEK (p-MEK) in detergent-soluble fractions and phosphorylated ERK (p-ERK) in detergent-insoluble fractions. p-ERK was colocalized and co-immunoprecipitated with occludin. GST (glutathione S-transferase) pull-down assay showed that the C-terminal tail of occludin binds to p-ERK in Caco-2 cell extracts. Pair-wise binding studies using recombinant proteins demonstrated that ERK1 directly interacts with the C-terminal tail of occludin. Therefore the present study shows that ERK interacts with the C-terminal region of occludin and mediates the prevention of H2O2-induced disruption of TJs by EGF.
Figures
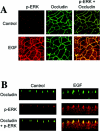
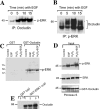

References
-
- Trier J. S. Celiac sprue and refractory sprue. In: Sleisenger M., Fordstran J., editors. Gastrointestinal Disease. Philadelphia: W. B. Saunders; 1978. pp. 1029–1051.
-
- Hollander D. Permeability in Crohn's disease: altered barrier functions in healthy relatives? Gastroenterology. 1993;104:1848–1851. - PubMed
-
- Mathay M. A., Fukuda N., Frank J., Kallet R., Daniel B., Sakuma T. Alveolar epithelial barrier. Role in lung fluid balance in clinical lung injury. Clin. Chest Med. 2000;21:477–490. - PubMed
-
- Anderson J. M., van Italie C. M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. - PubMed
-
- Tsukita S., Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

