Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth Syndrome
- PMID: 16135531
- PMCID: PMC1266419
- DOI: 10.1091/mbc.e05-03-0256
Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth Syndrome
Abstract
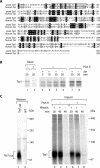
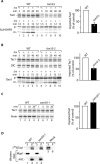
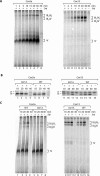
The Saccharomyces cerevisiae Taz1 protein is the orthologue of human Tafazzin, a protein that when inactive causes Barth Syndrome (BTHS), a severe inherited X-linked disease. Taz1 is a mitochondrial acyltransferase involved in the remodeling of cardiolipin. We show that Taz1 is an outer mitochondrial membrane protein exposed to the intermembrane space (IMS). Transport of Taz1 into mitochondria depends on the receptor Tom5 of the translocase of the outer membrane (TOM complex) and the small Tim proteins of the IMS, but is independent of the sorting and assembly complex (SAM). TAZ1 deletion in yeast leads to growth defects on nonfermentable carbon sources, indicative of a defect in respiration. Because cardiolipin has been proposed to stabilize supercomplexes of the respiratory chain complexes III and IV, we assess supercomplexes in taz1delta mitochondria and show that these are destabilized in taz1Delta mitochondria. This leads to a selective release of a complex IV monomer from the III2IV2 supercomplex. In addition, assembly analyses of newly imported subunits into complex IV show that incorporation of the complex IV monomer into supercomplexes is affected in taz1Delta mitochondria. We conclude that inactivation of Taz1 affects both assembly and stability of respiratory chain complexes in the inner membrane of mitochondria.
Figures


References
-
- Barth, P. G., Scholte, H. R., Berden, J. A., Van der Klei-Van Moorsel, J. M., Luyt-Houwen, I. E., Van't Veer-Korthof, E. T., Van der Harten, J. J., and Sobotka-Plojhar, M. A. (1983). An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 62, 327–355. - PubMed
-
- Barth, P. G., Valianpour, F., Bowen, V. M., Lam, J., Duran, M., Vaz, F. M., and Wanders, R. J. (2004). X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am. J. Med. Genet. 126, 349–354. - PubMed
-
- Barth, P. G., Van den Bogert, C., Bolhuis, P. A., Scholte, H. R., van Gennip, A. H., Schutgens, R. B., and Ketel, A. G. (1996). X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): respiratory-chain abnormalities in cultured fibroblasts. J. Inherit. Metab. Dis. 19, 157–160. - PubMed
-
- Barth, P. G., Wanders, R. J., and Vreken, P. (1999). X-linked cardioskeletal myopathy and neutropenia (Barth syndrome)—MIM 302060. J. Pediatr. 135, 273–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

