Gating reaction mechanisms for NMDA receptor channels
- PMID: 16135748
- PMCID: PMC6725445
- DOI: 10.1523/JNEUROSCI.1471-05.2005
Gating reaction mechanisms for NMDA receptor channels
Abstract
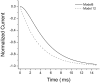
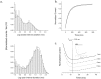
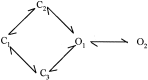
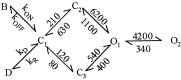
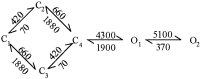
NMDA receptors (NMDARs) mediate the slow component of excitatory transmission in the CNS and play key roles in synaptic plasticity and excitotoxicity. We investigated the gating reaction mechanism of fully liganded NR1/NR2A recombinant NMDARs (expressed in Xenopus oocytes) by fitting all possible three-closed/two-open-state, noncyclic kinetic schemes to currents elicited by saturating concentrations of glutamate plus glycine. The adequacy of each scheme was assessed by maximum likelihood values and autocorrelation coefficients of single-channel currents, as well as by the predicted time courses of transient macroscopic currents. Two schemes provided the best description for NMDAR gating at both the single-channel and macroscopic levels. These two schemes had coupled open states, only one gateway between the closed and open aggregates, and at least two preopening closed states. These two models could be condensed into a cyclic reaction mechanism. Using a linear reaction scheme, the overall "gating" rates (from the initial stable closed state to the final stable open state) are 177 and 4.4 s(-1).
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
