Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models
- PMID: 16135751
- PMCID: PMC6725448
- DOI: 10.1523/JNEUROSCI.2467-05.2005
Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models
Abstract
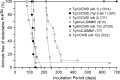
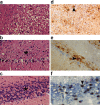
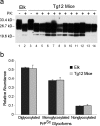
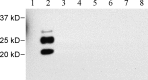
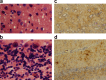
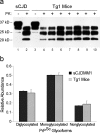
Chronic wasting disease (CWD), a prion disease affecting free-ranging and captive cervids (deer and elk), is widespread in the United States and parts of Canada. The large cervid population, the popularity of venison consumption, and the apparent spread of the CWD epidemic are likely resulting in increased human exposure to CWD in the United States. Whether CWD is transmissible to humans, as has been shown for bovine spongiform encephalopathy (the prion disease of cattle), is unknown. We generated transgenic mice expressing the elk or human prion protein (PrP) in a PrP-null background. After intracerebral inoculation with elk CWD prion, two lines of "humanized" transgenic mice that are susceptible to human prions failed to develop the hallmarks of prion diseases after >657 and >756 d, respectively, whereas the "cervidized" transgenic mice became infected after 118-142 d. These data indicate that there is a substantial species barrier for transmission of elk CWD to humans.
Figures
References
-
- Belay ED, Gambetti P, Schonberger LB, Parchi P, Lyon DR, Capellari S, McQuiston JH, Bradley K, Dowdle G, Crutcher JM, Nichol CR (2001) Creutzfeldt-Jakob disease in unusually young patients who consumed venison. Arch Neurol 58: 1673–1678. - PubMed
-
- Belay ED, Maddox RA, Gambetti P, Schonberger LB (2003) Monitoring the occurrence of emerging forms of Creutzfeldt-Jakob disease in the United States. Neurology 60: 176–181. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
