The histone H3 acetylase dGcn5 is a key player in Drosophila melanogaster metamorphosis
- PMID: 16135811
- PMCID: PMC1234334
- DOI: 10.1128/MCB.25.18.8228-8238.2005
The histone H3 acetylase dGcn5 is a key player in Drosophila melanogaster metamorphosis
Abstract
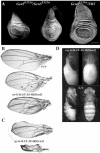
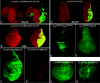
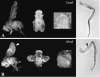
Although it has been well established that histone acetyltransferases (HATs) are involved in the modulation of chromatin structure and gene transcription, there is only little information on their developmental role in higher organisms. Gcn5 was the first transcription factor with HAT activity identified in eukaryotes. Here we report the isolation and characterization of Drosophila melanogaster dGcn5 mutants. Null dGcn5 alleles block the onset of both oogenesis and metamorphosis, while hypomorphic dGcn5 alleles impair the formation of adult appendages and cuticle. Strikingly, the dramatic loss of acetylation of the K9 and K14 lysine residues of histone H3 in dGcn5 mutants has no noticeable effect on larval tissues. In contrast, strong cell proliferation defects in imaginal tissues are observed. In vivo complementation experiments revealed that dGcn5 integrates specific functions in addition to chromosome binding and acetylation. Surprisingly, a dGcn5 variant protein with a deletion of the bromodomain, which has been shown to recognize acetylated histones, appears to be fully functional. Our results establish dGcn5 as a major histone H3 acetylase in Drosophila which plays a key role in the control of specific morphogenetic cascades during developmental transitions.
Figures




References
-
- Agalioti, T., G. Chen, and D. Thanos. 2002. Deciphering the transcriptional histone acetylation code for a human gene. Cell 111:381-392. - PubMed
-
- Agalioti, T., S. Lomvardas, B. Parekh, J. Yie, T. Maniatis, and D. Thanos. 2000. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell 103:667-678. - PubMed
-
- Ashburner, M., C. Chihara, P. Meltzer, and G. Richards. 1974. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harbor Symp. Quant. Biol. 38:655-662. - PubMed
-
- Bellaiche, Y., M. Gho, J. A. Kaltschmidt, A. H. Brand, and F. Schweisguth. 2001. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat. Cell Biol. 3:50-57. - PubMed
-
- Belyaeva, E. S., M. G. Aizenzon, V. F. Semeshin, I. I. Kiss, K. Koczka, E. M. Baritcheva, T. D. Gorelova, and I. F. Zhimulev. 1980. Cytogenetic analysis of the 2B3-4-2B11 region of the X-chromosome of Drosophila melanogaster. I. Cytology of the region and mutant complementation groups. Chromosoma 81:281-306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
