Pathway- and expression level-dependent effects of oncogenic N-Ras: p27(Kip1) mislocalization by the Ral-GEF pathway and Erk-mediated interference with Smad signaling
- PMID: 16135812
- PMCID: PMC1234306
- DOI: 10.1128/MCB.25.18.8239-8250.2005
Pathway- and expression level-dependent effects of oncogenic N-Ras: p27(Kip1) mislocalization by the Ral-GEF pathway and Erk-mediated interference with Smad signaling
Abstract
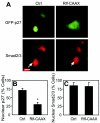
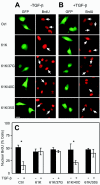
Overactivation of Ras pathways contributes to oncogenesis and metastasis of epithelial cells in several ways, including interference with cell cycle regulation via the CDK inhibitor p27(Kip1) (p27) and disruption of transforming growth factor beta (TGF-beta) anti-proliferative activity. Here, we show that at high expression levels, constitutively active N-Ras induces cytoplasmic mislocalization of murine and human p27 via the Ral-GEF pathway and disrupts TGF-beta-mediated Smad nuclear translocation by activation of the Mek/Erk pathway. While human p27 could also be mislocalized via the phosphatidylinositol 3-kinase/Akt pathway, only Ral-GEF activation was effective for murine p27, which lacks the Thr157 Akt phosphorylation site of human p27. This establishes a novel role for the Ral-GEF pathway in regulating p27 localization. Interference with either Smad translocation or p27 nuclear localization was sufficient to disrupt TGF-beta growth inhibition. Moreover, expression of activated N-Ras or specific effector loop mutants at lower levels using retroviral vectors induced p27 mislocalization but did not inhibit Smad2/3 translocation, indicating that the effects on p27 localization occur at lower levels of activated Ras. These findings have important implications for the contribution of activated Ras to oncogenesis and for the conversion of TGF-beta from an inhibitory to a metastatic factor in some epithelial tumors.
Figures





References
-
- Alexandrow, M. G., and H. L. Moses. 1995. Transforming growth factor β and cell cycle regulation. Cancer Res. 55:1452-1457. - PubMed
-
- Attisano, L., and J. L. Wrana. 2002. Signal transduction by the TGF-β superfamily. Science 296:1646-1647. - PubMed
-
- Baldassarre, G., B. Belletti, P. Bruni, A. Boccia, F. Trapasso, F. Pentimalli, M. V. Barone, G. Chiappetta, M. T. Vento, S. Spiezia, A. Fusco, and G. Viglietto. 1999. Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J. Clin. Investig. 104:865-874. - PMC - PubMed
-
- Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
