A guideline for analyzing circadian wheel-running behavior in rodents under different lighting conditions
- PMID: 16136228
- PMCID: PMC1190381
- DOI: 10.1251/bpo109
A guideline for analyzing circadian wheel-running behavior in rodents under different lighting conditions
Abstract
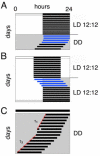
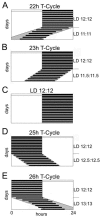
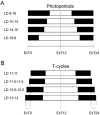
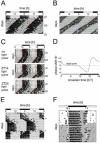
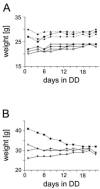
Most behavioral experiments within circadian research are based on the analysis of locomotor activity. This paper introduces scientists to chronobiology by explaining the basic terminology used within the field. Furthermore, it aims to assist in designing, carrying out, and evaluating wheel-running experiments with rodents, particularly mice. Since light is an easily applicable stimulus that provokes strong effects on clock phase, the paper focuses on the application of different lighting conditions.
Figures


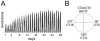
References
-
- Dunlap JC, Loros JL, DeCoursey PJ. CHRONOBIOLOGY – biological timekeeping. Sinauer Associates. 2004;3(24):67–105.
-
- Pittendrigh CS. On the mechanism of the entrainment of a circadian rhythm by light cycles. In: Circadian clocks; edited by: Aschoff J, Amsterdam: Elsevier (1965); 277-297.
-
- Pittendrigh CS. Circadian systems: entrainment. In: Handbook of behavioral neurobiology, Vol. 4 Biological Rhythms, edited by Aschoff J, New York: Plenum Press (1981); 95-124.
-
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol A. 1976;106:291–331.
-
- Aschoff J, Daan S, Honma KI. Zeitgeber, entrainment, and masking: some unsettled questions. In: Vertebrate Circadian System (Structure and Physiology), edited by Aschoff J, Daan S, Gross GA, Berlin: Springer-Verlag (1982); 13-24.
LinkOut - more resources
Full Text Sources
Other Literature Sources

