Study protocol: the DOse REsponse Multicentre International collaborative initiative (DO-RE-MI)
- PMID: 16137353
- PMCID: PMC1269446
- DOI: 10.1186/cc3718
Study protocol: the DOse REsponse Multicentre International collaborative initiative (DO-RE-MI)
Abstract
Introduction: Current practices for renal replacement therapy in intensive care units (ICUs) remain poorly defined. The DOse REsponse Multicentre International collaborative initiative (DO-RE-MI) will address the issue of how the different modes of renal replacement therapy are currently chosen and performed. Here, we describe the study protocol, which was approved by the Scientific and Steering Committees.
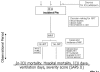
Methods: DO-RE-MI is an observational, multicentre study conducted in ICUs. The primary end-point will be the delivered dose of dialysis, which will be compared with ICU mortality, 28-day mortality, hospital mortality, ICU length of stay and number of days of mechanical ventilation. The secondary end-point will be the haemodynamic response to renal replacement therapy, expressed as percentage reduction in noradrenaline (norepinephrine) requirement. Based on the the sample analysis calculation, at least 162 patients must be recruited. Anonymized patient data will be entered online in electronic case report forms and uploaded to an internet website. Each participating centre will have 2 months to become acquainted with the electronic case report forms. After this period official recruitment will begin. Patient data belong to the respective centre, which may use the database for its own needs. However, all centres have agreed to participate in a joint effort to achieve the sample size needed for statistical analysis.
Conclusion: The study will hopefully help to collect useful information on the current practice of renal replacement therapy in ICUs. It will also provide a centre-based collection of data that will be useful for monitoring all aspects of extracorporeal support, such as incidence, frequency, and duration.
Figures