Differential detection of turkey coronavirus, infectious bronchitis virus, and bovine coronavirus by a multiplex polymerase chain reaction
- PMID: 16137773
- PMCID: PMC7112836
- DOI: 10.1016/j.jviromet.2005.07.007
Differential detection of turkey coronavirus, infectious bronchitis virus, and bovine coronavirus by a multiplex polymerase chain reaction
Abstract
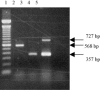
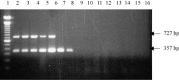
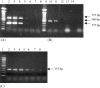
The objective of the present study was to develop a multiplex polymerase chain reaction (PCR) method for differential detection of turkey coronavirus (TCoV), infectious bronchitis coronavirus (IBV), and bovine coronavirus (BCoV). Primers were designed from conserved or variable regions of nucleocapsid (N) or spike (S) protein gene among TCoV, IBV, and BCoV and used in the same PCR reaction. Reverse transcription followed by the PCR reaction was used to amplify a portion of N or S gene of the corresponding coronaviruses. The PCR products were detected on agarose gel stained with ethidium bromide. Two PCR products, a 356-bp band corresponding to N gene and a 727-bp band corresponding to S gene, were obtained for TCoV isolates. In contrast, one PCR product of 356 bp corresponding to a fragment of N gene was obtained for IBV strains and one PCR product of 568 bp corresponding to a fragment of S gene was obtained for BCoV. There were no PCR products with the same primers for Newcastle disease virus, Marek's disease virus, turkey pox virus, pigeon pox virus, fowl pox virus, reovirus, infectious bursal disease virus, enterovirus, astrovirus, Salmonella enterica, Escherichia coli, and Mycoplasma gallisepticum. Performance of the assay with serially diluted RNA demonstrated that the multiplex PCR could detect 4.8x10(-3) microg of TCoV RNA, 4.6x10(-4) microg of IBV RNA, and 8.0x10(-2) microg of BCoV RNA. These results indicated that the multiplex PCR as established in the present study is a rapid, sensitive, and specific method for differential detection of TCoV, IBV, and BCoV in a single PCR reaction.
Figures

Similar articles
-
Antigenic and genomic relatedness of turkey-origin coronaviruses, bovine coronaviruses, and infectious bronchitis virus of chickens.Avian Dis. 2001 Oct-Dec;45(4):978-84. Avian Dis. 2001. PMID: 11785902
-
First full-length sequences of the S gene of European isolates reveal further diversity among turkey coronaviruses.Avian Pathol. 2011 Apr;40(2):179-89. doi: 10.1080/03079457.2011.551936. Avian Pathol. 2011. PMID: 21500038
-
Specific real-time reverse transcription-polymerase chain reaction for detection and quantitation of turkey coronavirus RNA in tissues and feces from turkeys infected with turkey coronavirus.J Virol Methods. 2010 Feb;163(2):452-8. doi: 10.1016/j.jviromet.2009.11.012. Epub 2009 Nov 14. J Virol Methods. 2010. PMID: 19917315 Free PMC article.
-
A reverse transcriptase-polymerase chain reaction assay for the diagnosis of turkey coronavirus infection.J Vet Diagn Invest. 2003 Nov;15(6):592-6. doi: 10.1177/104063870301500616. J Vet Diagn Invest. 2003. PMID: 14667027
-
What is the evidence that bovine coronavirus is a biologically significant respiratory pathogen in cattle?Can Vet J. 2019 Feb;60(2):147-152. Can Vet J. 2019. PMID: 30705449 Free PMC article. Review.
Cited by
-
Development of a multienzyme isothermal rapid amplification and lateral flow dipstick combination assay for bovine coronavirus detection.Front Vet Sci. 2023 Jan 4;9:1059934. doi: 10.3389/fvets.2022.1059934. eCollection 2022. Front Vet Sci. 2023. PMID: 36686176 Free PMC article.
-
Multiplex semi-nested RT-PCR with exogenous internal control for simultaneous detection of bovine coronavirus and group A rotavirus.J Virol Methods. 2010 Nov;169(2):375-9. doi: 10.1016/j.jviromet.2010.08.008. Epub 2010 Aug 17. J Virol Methods. 2010. PMID: 20723564 Free PMC article.
-
Pathogenic bacteria associated with outbreaks of respiratory disease in Iranian broiler farms.Vet Med Sci. 2023 Jul;9(4):1675-1684. doi: 10.1002/vms3.1162. Epub 2023 May 21. Vet Med Sci. 2023. PMID: 37210710 Free PMC article.
-
A novel multiplex PCR-electronic microarray assay for rapid and simultaneous detection of bovine respiratory and enteric pathogens.J Virol Methods. 2018 Nov;261:51-62. doi: 10.1016/j.jviromet.2018.08.010. Epub 2018 Aug 10. J Virol Methods. 2018. PMID: 30102924 Free PMC article.
-
Advances in Laboratory Diagnosis of Coronavirus Infections in Cattle.Pathogens. 2024 Jun 21;13(7):524. doi: 10.3390/pathogens13070524. Pathogens. 2024. PMID: 39057751 Free PMC article. Review.
References
-
- Akin A., Lin T.L., Wu C.C., Bryan T.A., Hooper T., Schrader D. Nucleocapsid protein gene sequence analysis reveals close genomic relationship between turkey coronavirus and avian infectious bronchitis virus. Acta Virol. 2001;45(1):31–38. - PubMed
-
- Akin A., Wu C.C., Lin T.L. Amplification and cloning of complete infectious bursal disease virus genomic RNA segments by a long and accurate PCR. J. Virol. Methods. 1999;82:55–61. - PubMed
-
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

