Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils
- PMID: 16138901
- PMCID: PMC2715425
- DOI: 10.1111/j.1600-0854.2005.00322.x
Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils
Abstract
Eosinophils, leukocytes involved in allergic, inflammatory and immunoregulatory responses, have a distinct capacity to rapidly secrete preformed granule-stored proteins through piecemeal degranulation (PMD), a secretion process based on vesicular transport of proteins from within granules for extracellular release. Eosinophil-specific granules contain cytokines and cationic proteins, such as major basic protein (MBP). We evaluated structural mechanisms responsible for mobilizing proteins from within eosinophil granules. Human eosinophils stimulated for 30-60 min with eotaxin, regulated on activation, normal, T-cell expressed and secreted (RANTES) or platelet activating factor exhibited ultrastructural features of PMD (e.g. losses of granule contents) and extensive vesiculotubular networks within emptying granules. Brefeldin A inhibited granule emptying and collapsed intragranular vesiculotubular networks. By immunonanogold ultrastructural labelings, CD63, a tetraspanin membrane protein, was localized within granules and on vesicles outside of granules, and mobilization of MBP into vesicles within and extending from granules was demonstrated. Electron tomography with three dimension reconstructions revealed granule internal membranes to constitute an elaborate tubular network able to sequester and relocate granule products upon stimulation. We provide new insights into PMD and identify eosinophil specific granules as organelles whose internal tubulovesicular networks are important for the capacity of eosinophils to secrete, by vesicular transport, their content of preformed and granule-stored cytokines and cationic proteins.
Figures

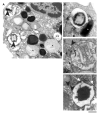

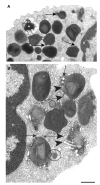
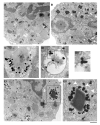

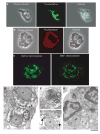

References
-
- Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–663. - PubMed
-
- Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. - PubMed
-
- Munitz A, Levi-Schaffer F. Eosinophils: ‘new’ roles for ‘old’ cells. Allergy. 2004;59:268–275. - PubMed
-
- Adamko DJ, Odemuyiwa SO, Vethanayagam D, Moqbel R. The rise of the phoenix: the expanding role of the eosinophil in health and disease. Allergy. 2005;60:13–22. - PubMed
-
- Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

