Coincidence detection in phosphoinositide signaling
- PMID: 16139503
- PMCID: PMC1904488
- DOI: 10.1016/j.tcb.2005.08.005
Coincidence detection in phosphoinositide signaling
Abstract
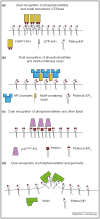
Phosphoinositide lipids function as both signaling molecules and as compartment-specific localization signals for phosphoinositide-binding proteins. In recent years, both phosphoinositides and phosphoinositide-binding proteins have been reported to display a restricted, rather than a uniform, distribution across intracellular membranes. Here, we examine recent data documenting the restricted distribution of both phosphoinositides and phosphoinositide-binding proteins and examine how phosphoinositide-binding proteins might engage multiple binding partners to achieve these restricted localizations, effectively acting as detectors of coincident localization signals.
Figures



References
-
- Cullen PJ, et al. Modular phosphoinositide-binding domains –their role in signalling and membrane trafficking. Curr Biol. 2001;11:R882–R893. - PubMed
-
- Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4:201–213. - PubMed
-
- Balla T. Inositol-lipid binding motifs: signal integrators through protein–lipid and protein–protein interactions. J Cell Sci. 2005;118:2093–2104. - PubMed
-
- Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol Rev. 2004;84:699–730. - PubMed
-
- Kobayashi T, et al. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

