Mechanism of membrane fusion by viral envelope proteins
- PMID: 16139596
- PMCID: PMC7173036
- DOI: 10.1016/S0065-3527(05)64007-9
Mechanism of membrane fusion by viral envelope proteins
Abstract
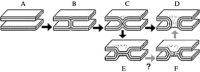
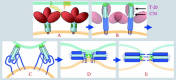
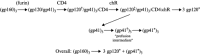
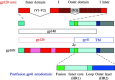
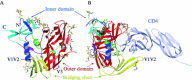
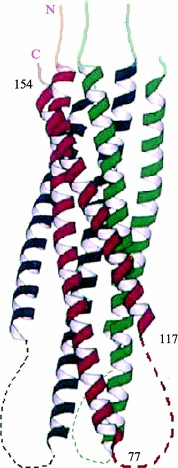
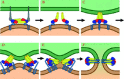
Enveloped viruses enter cells by fusing their lipid bilayer membrane with a cellular membrane. Most viral fusion proteins require priming by proteolytic processing, either of the fusion protein itself or of an accompanying protein. The priming step, which often occurs during transport of the fusion protein to the cell surface but may also occur extracellularly, then prepares the fusion protein for triggering by events that accompany attachment and uptake. Two classes of viral fusion proteins have been identified so far by structural studies. The fusion of two bilayers that these proteins catalyze is likely to proceed by the same pathway in both cases. That is, these proteins are like enzymes that have different structures but that still catalyze the same chemical reaction. It is found that bilayer fusion reaction is common to all the enveloped viral entry pathways. It is believed to pass through an intermediate known as a “hemifusion stalk.”
Figures

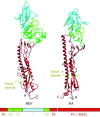


References
-
- Allan J.S., Coligan J.E., Barin F., McLane M.F., Sodroski J.G., Rosen C.A., Haseltine W.A., Lee T.H., Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV‐III. Science. 1985;228:1091–1094. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

