Sialidase activity of influenza A virus in an endocytic pathway enhances viral replication
- PMID: 16140748
- PMCID: PMC1212606
- DOI: 10.1128/JVI.79.18.11705-11715.2005
Sialidase activity of influenza A virus in an endocytic pathway enhances viral replication
Abstract
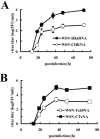
N2 neuraminidase (NA) genes of the 1957 and 1968 pandemic influenza virus strains possessed avian-like low-pH stability of sialidase activity, unlike most epidemic strains. We generated four reverse-genetics viruses from a genetic background of A/WSN/33 (H1N1) that included parental N2 NAs of 1968 pandemic (H3N2) and epidemic (H2N2) strains or their counterpart N2 NAs in which the low-pH stability of the sialidase activity was changed by substitutions of one or two amino acid residues. We found that the transfectant viruses bearing low-pH-stable sialidase (WSN/Stable-NAs) showed 25- to 80-times-greater ability to replicate in Madin-Darby canine kidney (MDCK) cells than did the transfectant viruses bearing low-pH-unstable sialidase (WSN/Unstable-NAs). Enzymatic activities of WSN/Stable-NAs were detected in endosomes of MDCK cells after 90 min of virus internalization by in situ fluorescent detection with 5-bromo-4-chloro-indole-3-yl-alpha-N-acetylneuraminic acid and Fast Red Violet LB. Inhibition of sialidase activity of WSN/Stable-NAs on the endocytic pathway by pretreatment with 4-guanidino-2,4-dideoxy-N-acetylneuraminic acid (zanamivir) resulted in a significant decrease in progeny viruses. In contrast, the enzymatic activities of WSN/Unstable-NAs, the replication of which had no effect on pretreatment with zanamivir, were undetectable in cells under the same conditions. Hemadsorption assays of transfectant-virus-infected cells revealed that the low-pH stability of the sialidase had no effect on the process of removal of sialic acid from hemagglutinin in the Golgi regions. Moreover, high titers of viruses were recovered from the lungs of mice infected with WSN/Stable-NAs on day 3 after intranasal inoculation, but WSN/Unstable-NAs were cleared from the lungs of the mice. These results indicate that sialidase activity in late endosome/lysosome traffic enhances influenza A virus replication.
Figures




References
-
- Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. - PubMed
-
- Henkel, J. R., and O. A. Weisz. 1988. Influenza virus M2 protein slows traffic along the secretory pathway. pH perturbation of acidified compartments affects early Golgi transport steps. J. Biol. Chem. 273:6518-6524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

