Rab9 GTPase is required for replication of human immunodeficiency virus type 1, filoviruses, and measles virus
- PMID: 16140752
- PMCID: PMC1212642
- DOI: 10.1128/JVI.79.18.11742-11751.2005
Rab9 GTPase is required for replication of human immunodeficiency virus type 1, filoviruses, and measles virus
Abstract
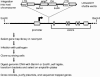
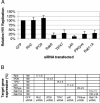
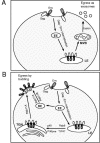
Rab proteins and their effectors facilitate vesicular transport by tethering donor vesicles to their respective target membranes. By using gene trap insertional mutagenesis, we identified Rab9, which mediates late-endosome-to-trans-Golgi-network trafficking, among several candidate host genes whose disruption allowed the survival of Marburg virus-infected cells, suggesting that Rab9 is utilized in Marburg replication. Although Rab9 has not been implicated in human immunodeficiency virus (HIV) replication, previous reports suggested that the late endosome is an initiation site for HIV assembly and that TIP47-dependent trafficking out of the late endosome to the trans-Golgi network facilitates the sorting of HIV Env into virions budding at the plasma membrane. We examined the role of Rab9 in the life cycles of HIV and several unrelated viruses, using small interfering RNA (siRNA) to silence Rab9 expression before viral infection. Silencing Rab9 expression dramatically inhibited HIV replication, as did silencing the host genes encoding TIP47, p40, and PIKfyve, which also facilitate late-endosome-to-trans-Golgi vesicular transport. In addition, silencing studies revealed that HIV replication was dependent on the expression of Rab11A, which mediates trans-Golgi-to-plasma-membrane transport, and that increased HIV Gag was sequestered in a CD63+ endocytic compartment in a cell line stably expressing Rab9 siRNA. Replication of the enveloped Ebola, Marburg, and measles viruses was inhibited with Rab9 siRNA, although the non-enveloped reovirus was insensitive to Rab9 silencing. These results suggest that Rab9 is an important cellular target for inhibiting diverse viruses and help to define a late-endosome-to-plasma-membrane vesicular transport pathway important in viral assembly.
Figures

References
-
- Basyuk, E., T. Galli, M. Mougel, J. M. Blanchard, M. Sitbon, and E. Bertrand. 2003. Retroviral genomic RNAs are transported to the plasma membrane by endosomal vesicles. Dev. Cell 5:161-174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

