Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones
- PMID: 16141270
- PMCID: PMC1464206
- DOI: 10.1113/jphysiol.2005.095042
Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones
Abstract
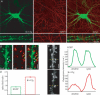
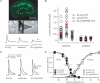
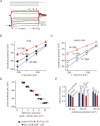
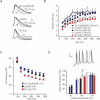
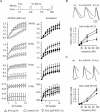
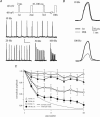
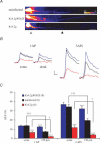
A-type potassium channels regulate neuronal firing frequency and the back-propagation of action potentials (APs) into dendrites of hippocampal CA1 pyramidal neurones. Recent molecular cloning studies have found several families of voltage-gated K(+) channel genes expressed in the mammalian brain. At present, information regarding the relationship between the protein products of these genes and the various neuronal functions performed by voltage-gated K(+) channels is lacking. Here we used a combination of molecular, electrophysiological and imaging techniques to show that one such gene, Kv4.2, controls AP half-width, frequency-dependent AP broadening and dendritic action potential propagation. Using a modified Sindbis virus, we expressed either the enhanced green fluorescence protein (EGFP)-tagged Kv4.2 or an EGFP-tagged dominant negative mutant of Kv4.2 (Kv4.2g(W362F)) in CA1 pyramidal neurones of organotypic slice cultures. Neurones expressing Kv4.2g(W362F) displayed broader action potentials with an increase in frequency-dependent AP broadening during a train compared with control neurones. In addition, Ca(2)(+) imaging of Kv4.2g(W362F) expressing dendrites revealed enhanced AP back-propagation compared to control neurones. Conversely, neurones expressing an increased A-type current through overexpression of Kv4.2 displayed narrower APs with less frequency dependent broadening and decreased dendritic propagation. These results point to Kv4.2 as the major contributor to the A-current in hippocampal CA1 neurones and suggest a prominent role for Kv4.2 in regulating AP shape and dendritic signalling. As Ca(2)(+) influx occurs primarily during AP repolarization, Kv4.2 activity can regulate cellular processes involving Ca(2)(+)-dependent second messenger cascades such as gene expression and synaptic plasticity.
Figures

References
-
- Alonso G, Widmer H. Clustering of KV4.2 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. - PubMed
-
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
-
- Anderson AE, Adams JP, Qian Y, Cook RG, Pfaffinger PJ, Sweatt JD. Kv4.2 phosphorylation by cyclic AMP-dependent protein kinase. J Biol Chem. 2000;275:5337–5346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

