The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study
- PMID: 16141345
- PMCID: PMC1895343
- DOI: 10.1182/blood-2005-05-2049
The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study
Abstract
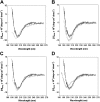
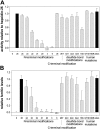
Hepcidin is the principal iron-regulatory hormone. It acts by binding to the iron exporter ferroportin, inducing its internalization and degradation, thereby blocking cellular iron efflux. The bioactive 25 amino acid (aa) peptide has a hairpin structure stabilized by 4 disulfide bonds. We synthesized a series of hepcidin derivatives and determined their bioactivity in a cell line expressing ferroportin-GFP fusion protein, by measuring the degradation of ferroportin-GFP and the accumulation of ferritin after peptide treatment. Bioactivity was also assayed in mice by the induction of hypoferremia. Serial deletion of N-terminal amino acids caused progressive decrease in activity which was completely lost when 5 N-terminal aa's were deleted. Synthetic 3-aa and 6-aa N-terminal peptides alone, however, did not internalize ferroportin and did not interfere with ferroportin internalization by native hepcidin. Deletion of 2 C-terminal aa's did not affect peptide activity. Removal of individual disulfide bonds by pairwise substitution of cysteines with alanines also did not affect peptide activity in vitro. However, these peptides were less active in vivo, likely because of their decreased stability in circulation. G71D and K83R, substitutions previously described in humans, did not affect hepcidin activity. Apart from the essential nature of the N-terminus, hepcidin structure appears permissive for mutations.
Figures


References
-
- Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276: 7811-7819. - PubMed
-
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306: 2090-2093. - PubMed
-
- Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1: 191-200. - PubMed
-
- Weinstein DA, Roy CN, Fleming MD, et al. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100: 3776-3781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

