Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines
- PMID: 16141352
- PMCID: PMC1895342
- DOI: 10.1182/blood-2005-05-2023
Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines
Abstract
Efficient bone marrow (BM) homing is a prerequisite for successful engraftment of transplanted hematopoietic cells (HPCs). Contradictory conclusions about the contribution of SDF-1/CXCR4 have clouded our understanding of its role within the molecular pathway cooperation needed for BM homing, particularly with the well-defined hierarchic network of adhesion molecules. In the present study we sought to unravel cooperative and compensatory molecular pathways guiding BM homing. Fresh BM-HPCs, rendered either SDF-1 unresponsive or Gi-signaling refractory, homed quite efficiently, because of compensation by alpha4-integrin interacting with VCAM-1. The contribution of SDF-1/CXCR4- or Gi-protein-mediated signals to BM homing became apparent after their blockade was combined with deletion of alpha4-integrin, leading to dramatic reduction in BM homing. Similar conclusions were revealed when VCAM-1-deficient hosts were used. Cytokine incubation changed the functional properties of BM-HPCs and hierarchy of molecular pathway usage in homing, by shifting the dominance among the homing mediators: loss of CXCR4 or Gi-signaling now significantly reduced BM homing, with only partial compensation through alpha4/VCAM-1 and endothelial selectins. These studies depict a flexible hierarchy of cooperating homing pathways, in which dominant players are repositioned with changing cytokine milieu, and possibly source of HPCs.
Figures
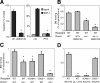
 ) or chemotactic (SDF-1, ▪) migration of fresh c-kit+ WT BM-HPCs through 5-μm transwells was tested in untreated control BM cells, in the presence of AMD3100 (100 μg/mL) or after PTX treatment (100 ng/mL, 4 hours). AMD3100 (left) significantly attenuated SDF-1-directed migration. PTX incubation (right) completely blocked SDF-1-directed migration, but low-level spontaneous migration was maintained in the presence of PTX (*P < .05). (B-D) BM homing of fresh BM-HPCs was tested 3 hours (B) or 18 hours (C,D) after transplantation of lethally irradiated recipients. Donor-cell strain/treatment and recipient strain are indicated below the respective bars. BM homing is given as the percentage of injected CFU-Cs recovered per femur. Depicted are mean plus SEM of all mice tested with this modality. (B) Three-hour BM homing of fresh BM cells treated with or without AMD3100. BM homing of fresh WT BM cells was not affected by AMD3100 incubation/coinjection. BM homing of α4-/- BM cells in WT recipients was significantly decreased compared with WT-to-WT (*P < .05). AMD3100 treatment of α4-/- BM cells additionally reduced BM homing (*P < .05 compared with untreated α4-/--to-WT transplantation). (C) Eighteen-hour BM homing of fresh BM cells. BM homing of α4-/- cells in WT recipients, or WT cells in VCAM-1-/- recipients was significantly reduced compared with WT-to-WT (*P < .05). BM homing of WT BM in CD62-/- recipients, which are deficient in endothelial selectins, was no different from WT-to-WT. BM homing of α4-/- BM-HPCs in CD62-/- recipients was significantly less efficient than that of α4-/- in WT (*P < .05). (D) Eighteen-hour BM homing of fresh PTX-treated BM cells. BM homing of PTX-treated WT cells in WT recipients was no different from untreated WT-to-WT, but BM homing of PTX-treated α4-/- cells in WT recipients, or BM homing of PTX-treated WT cells in VCAM-1-/- hosts were almost completely abrogated (*P < .05 compared with untreated α4-/--to-WT or WT-to-VCAM-1 transplantation). In contrast, PTX-treated WT cells homed normally in CD62-/- hosts.
) or chemotactic (SDF-1, ▪) migration of fresh c-kit+ WT BM-HPCs through 5-μm transwells was tested in untreated control BM cells, in the presence of AMD3100 (100 μg/mL) or after PTX treatment (100 ng/mL, 4 hours). AMD3100 (left) significantly attenuated SDF-1-directed migration. PTX incubation (right) completely blocked SDF-1-directed migration, but low-level spontaneous migration was maintained in the presence of PTX (*P < .05). (B-D) BM homing of fresh BM-HPCs was tested 3 hours (B) or 18 hours (C,D) after transplantation of lethally irradiated recipients. Donor-cell strain/treatment and recipient strain are indicated below the respective bars. BM homing is given as the percentage of injected CFU-Cs recovered per femur. Depicted are mean plus SEM of all mice tested with this modality. (B) Three-hour BM homing of fresh BM cells treated with or without AMD3100. BM homing of fresh WT BM cells was not affected by AMD3100 incubation/coinjection. BM homing of α4-/- BM cells in WT recipients was significantly decreased compared with WT-to-WT (*P < .05). AMD3100 treatment of α4-/- BM cells additionally reduced BM homing (*P < .05 compared with untreated α4-/--to-WT transplantation). (C) Eighteen-hour BM homing of fresh BM cells. BM homing of α4-/- cells in WT recipients, or WT cells in VCAM-1-/- recipients was significantly reduced compared with WT-to-WT (*P < .05). BM homing of WT BM in CD62-/- recipients, which are deficient in endothelial selectins, was no different from WT-to-WT. BM homing of α4-/- BM-HPCs in CD62-/- recipients was significantly less efficient than that of α4-/- in WT (*P < .05). (D) Eighteen-hour BM homing of fresh PTX-treated BM cells. BM homing of PTX-treated WT cells in WT recipients was no different from untreated WT-to-WT, but BM homing of PTX-treated α4-/- cells in WT recipients, or BM homing of PTX-treated WT cells in VCAM-1-/- hosts were almost completely abrogated (*P < .05 compared with untreated α4-/--to-WT or WT-to-VCAM-1 transplantation). In contrast, PTX-treated WT cells homed normally in CD62-/- hosts.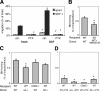
 ) and SDF-1-directed chemotactic migration (▪) of fresh or overnight SCF-incubated cells, with or without PTX, was quantified after 4 hours. Depicted are mean plus SEM. Spontaneous and SDF-1-directed migration were both significantly increased by SCF incubation. This increase in migration was completely blocked by PTX, to levels of spontaneous migration of fresh BM, which apparently represents Gi-protein-independent migration (*P < .05). (B-D) BM homing of overnight SCF-incubated BM-HPCs. BM homing of SCF-incubated BM-HPCs was tested 3 hours (B) or 18 hours (C,D) after transplantation of lethally irradiated recipients. Donor-cell source/treatment and recipient strain are indicated below the respective bars. BM homing is given as percentage of injected CFU-Cs recovered per femur. Depicted are mean plus SEM of all mice tested with this donor/host constellation. (B) Three-hour BM homing of SCF-incubated cells treated with or without AMD3100. BM homing of fresh WT BM cells was significantly reduced by AMD3100 incubation/coinjection (*P < .05). (C) Eighteen-hour BM homing of SCF-incubated BM cells. BM homing of α4-/- cells in WT recipients was significantly reduced compared with WT-to-WT (*P < .05). BM homing of WT cells in CD62-/- recipients or of β2-/- cells in WT recipients were no different from WT-to-WT. (D) Eighteen-hour BM homing of SCF plus PTX-treated BM cells. BM homing of SCF plus PTX-treated WT cells in WT recipients was 75% reduced compared with SCF-treated WT-to-WT without PTX (P < .05). BM homing of SCF plus PTX-treated α4-/- cells in WT recipients or BM homing of SCF plus PTX-treated WT cells in VCAM-1-/- hosts was almost completely abrogated, significantly more strongly than SCF plus PTX-treated WT-to-WT (*P < .05). The inhibition of homing of SCF plus PTX-incubated β2-/- cells in WT recipients was similar to that of SCF plus PTX-treated WT donor cells.
) and SDF-1-directed chemotactic migration (▪) of fresh or overnight SCF-incubated cells, with or without PTX, was quantified after 4 hours. Depicted are mean plus SEM. Spontaneous and SDF-1-directed migration were both significantly increased by SCF incubation. This increase in migration was completely blocked by PTX, to levels of spontaneous migration of fresh BM, which apparently represents Gi-protein-independent migration (*P < .05). (B-D) BM homing of overnight SCF-incubated BM-HPCs. BM homing of SCF-incubated BM-HPCs was tested 3 hours (B) or 18 hours (C,D) after transplantation of lethally irradiated recipients. Donor-cell source/treatment and recipient strain are indicated below the respective bars. BM homing is given as percentage of injected CFU-Cs recovered per femur. Depicted are mean plus SEM of all mice tested with this donor/host constellation. (B) Three-hour BM homing of SCF-incubated cells treated with or without AMD3100. BM homing of fresh WT BM cells was significantly reduced by AMD3100 incubation/coinjection (*P < .05). (C) Eighteen-hour BM homing of SCF-incubated BM cells. BM homing of α4-/- cells in WT recipients was significantly reduced compared with WT-to-WT (*P < .05). BM homing of WT cells in CD62-/- recipients or of β2-/- cells in WT recipients were no different from WT-to-WT. (D) Eighteen-hour BM homing of SCF plus PTX-treated BM cells. BM homing of SCF plus PTX-treated WT cells in WT recipients was 75% reduced compared with SCF-treated WT-to-WT without PTX (P < .05). BM homing of SCF plus PTX-treated α4-/- cells in WT recipients or BM homing of SCF plus PTX-treated WT cells in VCAM-1-/- hosts was almost completely abrogated, significantly more strongly than SCF plus PTX-treated WT-to-WT (*P < .05). The inhibition of homing of SCF plus PTX-incubated β2-/- cells in WT recipients was similar to that of SCF plus PTX-treated WT donor cells.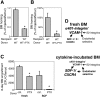
References
-
- Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98: 2403-2411. - PubMed
-
- Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103: 2981-2989. - PubMed
-
- Kimura T, Boehmler AM, Seitz G, et al. The sphingosine 1-phosphate (S1P) receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103: 4478-4486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

