TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing
- PMID: 16142218
- PMCID: PMC1369185
- DOI: 10.1038/sj.embor.7400509
TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing
Abstract
Dicer is a key enzyme involved in RNA interference (RNAi) and microRNA (miRNA) pathways. It is required for biogenesis of miRNAs and small interfering RNAs (siRNAs), and also has a role in the effector steps of RNA silencing. Apart from Argonautes, no proteins are known to associate with Dicer in mammalian cells. In this work, we describe the identification of TRBP (human immunodeficiency virus (HIV-1) transactivating response (TAR) RNA-binding protein) as a protein partner of human Dicer. We show that TRBP is required for optimal RNA silencing mediated by siRNAs and endogenous miRNAs, and that it facilitates cleavage of pre-miRNA in vitro. TRBP had previously been assigned several functions, including inhibition of the interferon-induced double-stranded RNA-regulated protein kinase PKR and modulation of HIV-1 gene expression by association with TAR. The TRBP-Dicer interaction shown raises interesting questions about the potential interplay between RNAi and interferon-PKR pathways.
Figures




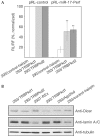
References
-
- Bannwarth S, Talakoub L, Letourneur F, Duarte M, Purcell DF, Hiscott J, Gatignol A (2001) Organization of the human tarbp2 gene reveals two promoters that are repressed in an astrocytic cell line. J Biol Chem 276: 48803–48813 - PubMed
-
- Bennasser Y, Le SY, Benkirane M, Jeang KT (2005) Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity 22: 607–619 - PubMed
-
- Bennett RL, Blalock WL, May WS (2004) Serine 18 phosphorylation of RAX, the PKR activator, is required for PKR activation and consequent translation inhibition. J Biol Chem 279: 42687–42693 - PubMed
-
- Bernstein E et al. (2003) Dicer is essential for mouse development. Nat Genet 35: 215–217 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

