The melanocyte differentiation program predisposes to metastasis after neoplastic transformation
- PMID: 16142232
- PMCID: PMC1694635
- DOI: 10.1038/ng1634
The melanocyte differentiation program predisposes to metastasis after neoplastic transformation
Abstract
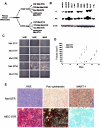
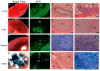
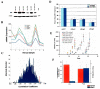
The aggressive clinical behavior of melanoma suggests that the developmental origins of melanocytes in the neural crest might be relevant to their metastatic propensity. Here we show that primary human melanocytes, transformed using a specific set of introduced genes, form melanomas that frequently metastasize to multiple secondary sites, whereas human fibroblasts and epithelial cells transformed using an identical set of genes generate primary tumors that rarely do so. Notably, these melanomas have a metastasis spectrum similar to that observed in humans with melanoma. These observations indicate that part of the metastatic proclivity of melanoma is attributable to lineage-specific factors expressed in melanocytes and not in other cell types analyzed. Analysis of microarray data from human nevi shows that the expression pattern of Slug, a master regulator of neural crest cell specification and migration, correlates with those of other genes that are important for neural crest cell migrations during development. Moreover, Slug is required for the metastasis of the transformed melanoma cells. These findings indicate that melanocyte-specific factors present before neoplastic transformation can have a pivotal role in governing melanoma progression.
Figures

References
-
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–85. - PubMed
-
- Beddingfield FC., 3rd The melanoma epidemic: res ipsa loquitur. Oncologist. 2003;8:459–65. - PubMed
-
- Bartkova J, et al. The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res. 1996;56:5475–83. - PubMed
-
- Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483–8. - PubMed
-
- Chin L. The genetics of malignant melanoma: lessons from mouse and man. Nat Rev Cancer. 2003;3:559–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
