Reading embossed capital letters: an fMRI study in blind and sighted individuals
- PMID: 16142777
- PMCID: PMC3684958
- DOI: 10.1002/hbm.20188
Reading embossed capital letters: an fMRI study in blind and sighted individuals
Abstract
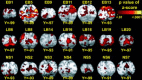
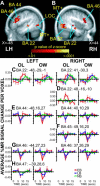
Reading Braille activates visual cortex in blind people [Burton et al., J Neurophysiol 2002;87:589-611; Sadato et al., Nature 1996;380:526-528; Sadato et al., Brain 1998;121:1213-1229]. Because learning Braille requires extensive training, we had sighted and blind people read raised block capital letters to determine whether all groups engage visual cortex similarly when reading by touch. Letters were passively rubbed across the right index finger at 30 mm/s using an MR-compatible drum stimulator. Age-matched sighted, early blind (lost sight 0-5 years), and late blind (lost sight >5.5 years) volunteers performed three tasks: stating an identified letter, stating a verb containing an identified letter, and feeling a moving smooth surface. Responses were voiced immediately after the drum stopped moving across the fingertip. All groups showed increased activity in visual areas V1 and V2 during both letter identification tasks. Blind compared to sighted participants showed greater activation increases predominantly in the parafoveal-peripheral portions of visuotopic areas and posterior parts of BA 20 and 37. Sighted participants showed suppressed activity in most of the same areas except for small positive responses bilaterally in V1, left V5/MT+, and bilaterally in BA 37/20. Blind individuals showed suppression of the language areas in the frontal cortex, while sighted individuals showed slight positive responses. Early blind showed a more extensive distribution of activity in superior temporal sulcal multisensory areas. These results show cross-modal reorganization of visual cortex and altered response dynamics in nonvisual areas that plausibly reflect mechanisms for adaptive plasticity in blindness.
Reading Braille activates visual cortex in blind people [Burton et al., J Neurophysiol 2002;87:589–611; Sadato et al., Nature 1996;380:526–528; Sadato et al., Brain 1998;121:1213–1229]. Because learning Braille requires extensive training, we had sighted and blind people read raised block capital letters to determine whether all groups engage visual cortex similarly when reading by touch. Letters were passively rubbed across the right index finger at 30 mm/s using an MR‐compatible drum stimulator. Age‐matched sighted, early blind (lost sight 0–5 years), and late blind (lost sight >5.5 years) volunteers performed three tasks: stating an identified letter, stating a verb containing an identified letter, and feeling a moving smooth surface. Responses were voiced immediately after the drum stopped moving across the fingertip. All groups showed increased activity in visual areas V1 and V2 during both letter identification tasks. Blind compared to sighted participants showed greater activation increases predominantly in the parafoveal‐peripheral portions of visuotopic areas and posterior parts of BA 20 and 37. Sighted participants showed suppressed activity in most of the same areas except for small positive responses bilaterally in V1, left V5/MT+, and bilaterally in BA 37/20. Blind individuals showed suppression of the language areas in the frontal cortex, while sighted individuals showed slight positive responses. Early blind showed a more extensive distribution of activity in superior temporal sulcal multisensory areas. These results show cross‐modal reorganization of visual cortex and altered response dynamics in nonvisual areas that plausibly reflect mechanisms for adaptive plasticity in blindness. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Figures




Similar articles
-
Adaptive changes in early and late blind: a FMRI study of verb generation to heard nouns.J Neurophysiol. 2002 Dec;88(6):3359-71. doi: 10.1152/jn.00129.2002. J Neurophysiol. 2002. PMID: 12466452 Free PMC article.
-
Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals.Hum Brain Mapp. 2004 Dec;23(4):210-28. doi: 10.1002/hbm.20064. Hum Brain Mapp. 2004. PMID: 15449356 Free PMC article. Clinical Trial.
-
Adaptive changes in early and late blind: a fMRI study of Braille reading.J Neurophysiol. 2002 Jan;87(1):589-607. doi: 10.1152/jn.00285.2001. J Neurophysiol. 2002. PMID: 11784773 Free PMC article. Clinical Trial.
-
How the blind "see" Braille: lessons from functional magnetic resonance imaging.Neuroscientist. 2005 Dec;11(6):577-82. doi: 10.1177/1073858405277314. Neuroscientist. 2005. PMID: 16282598 Review.
-
Cross-modal plasticity of tactile perception in blindness.Restor Neurol Neurosci. 2010;28(2):271-81. doi: 10.3233/RNN-2010-0534. Restor Neurol Neurosci. 2010. PMID: 20404414 Free PMC article. Review.
Cited by
-
Beat Detection Recruits the Visual Cortex in Early Blind Subjects.Life (Basel). 2021 Mar 31;11(4):296. doi: 10.3390/life11040296. Life (Basel). 2021. PMID: 33807372 Free PMC article.
-
A thalamocortical pathway for fast rerouting of tactile information to occipital cortex in congenital blindness.Nat Commun. 2019 Nov 14;10(1):5154. doi: 10.1038/s41467-019-13173-7. Nat Commun. 2019. PMID: 31727882 Free PMC article.
-
Right occipital cortex activation correlates with superior odor processing performance in the early blind.PLoS One. 2013 Aug 14;8(8):e71907. doi: 10.1371/journal.pone.0071907. eCollection 2013. PLoS One. 2013. PMID: 23967263 Free PMC article.
-
Recognition memory for vibrotactile rhythms: an fMRI study in blind and sighted individuals.Somatosens Mot Res. 2011;28(3-4):48-62. doi: 10.3109/08990220.2011.602765. Epub 2011 Aug 17. Somatosens Mot Res. 2011. PMID: 21846300 Free PMC article.
-
Multisensory mental imagery of fatigue: Evidence from an fMRI study.Hum Brain Mapp. 2022 Jul;43(10):3143-3152. doi: 10.1002/hbm.25839. Epub 2022 Mar 22. Hum Brain Mapp. 2022. PMID: 35315967 Free PMC article.
References
-
- Aleman A, van Lee L, Mantione MH, Verkoijen IG, de Haan EH (2001): Visual imagery without visual experience: evidence from congenitally totally blind people. Neuroreport 12: 2601–2604. - PubMed
-
- Amedi A, Raz N, Pianka P, Malach R, Zohary E (2003): Early 'visual' cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci 6: 758–766. - PubMed
-
- Andersson JL, Sundin A, Valind S (1995): A method for coregistration of PET and MR brain images. J Nucl Med 36: 1307–1315. - PubMed
-
- Arno P, De Volder AG, Vanlierde A, Wanet‐Defalque MC, Streel E, Robert A, Sanabria‐Bohorquez S, Veraart C (2001): Occipital activation by pattern recognition in the early blind using auditory substitution for vision. Neuroimage 13: 632–645. - PubMed
-
- Beauchamp MS, Argall BD, Bodurka J, Duyn JH, Martin A (2004a): Unraveling multisensory integration: patchy organization within human STS multisensory cortex. Nat Neurosci 7: 1190–1192. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

