The HhH2/NDD domain of the Drosophila Nod chromokinesin-like protein is required for binding to chromosomes in the oocyte nucleus
- PMID: 16143607
- PMCID: PMC1456107
- DOI: 10.1534/genetics.105.047464
The HhH2/NDD domain of the Drosophila Nod chromokinesin-like protein is required for binding to chromosomes in the oocyte nucleus
Abstract
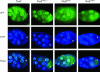
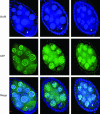
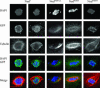
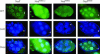
Nod is a chromokinesin-like protein that plays a critical role in segregating achiasmate chromosomes during female meiosis. The C-terminal half of the Nod protein contains two putative DNA-binding domains. The first of these domains, known as the HMGN domain, consists of three tandemly repeated high-mobility group N motifs. This domain was previously shown to be both necessary and sufficient for binding of the C-terminal half of Nod to mitotic chromosomes in embryos. The second putative DNA-binding domain, denoted HhH(2)/NDD, is a helix-hairpin-helix(2)/Nod-like DNA-binding domain. Although the HhH(2)/NDD domain is not required or sufficient for chromosome binding in embryos, several well-characterized nod mutations have been mapped in this domain. To characterize the role of the HhH(2)/NDD domain in mediating Nod function, we created a series of UAS-driven transgene constructs capable of expressing either a wild-type Nod-GFP fusion protein or proteins in which the HhH(2)/NDD domain had been altered by site-directed mutagenesis. Although wild-type Nod-GFP localizes to the oocyte chromosomes and rescues the segregation defect in nod mutant oocytes, two of three proteins carrying mutants in the HhH(2)/NDD domain fail to either rescue the nod mutant phenotype or bind to oocyte chromosomes. However, these mutant proteins do bind to the polytene chromosomes in nurse-cell nuclei and enter the oocyte nucleus. Thus, even though the HhH(2)/NDD domain is not essential for chromosome binding in other cell types, it is required for chromosome binding in the oocyte. These HhH(2)/NDD mutants also block the localization of Nod to the posterior pole of stage 9-10A oocytes, a process that is thought to facilitate the interaction of Nod with the plus ends of microtubules (Cui et al. 2005). This observation suggests that the Nod HhH2/NDD domain may play other roles in addition to binding Nod to meiotic chromosomes.
Figures



References
-
- Afshar, K., N. Barton, R. S. Hawley and L. S. B. Goldstein, 1995. a DNA binding and meiotic chromosome localization of the Drosophila NOD kinesin-like protein. Cell 81: 129–138. - PubMed
-
- Arn, E. A., B. J. Cha, W. E. Theurkauf and P. M. Macdonald, 2003. Recognition of a bicoid mRNA localization signal by a protein complex containing Swallow, Nod, and RNA binding proteins. Dev. Cell 4: 41–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

