Electron transfer versus proton transfer in gas-phase ion/ion reactions of polyprotonated peptides
- PMID: 16144411
- PMCID: PMC1570753
- DOI: 10.1021/ja0526057
Electron transfer versus proton transfer in gas-phase ion/ion reactions of polyprotonated peptides
Abstract
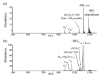
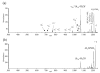
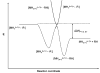
The ion/ion reactions of several dozen reagent anions with triply protonated cations of the model peptide KGAILKGAILR have been examined to evaluate predictions of a Landau-Zener-based model for the likelihood for electron transfer. Evidence for electron transfer was provided by the appearance of fragment ions unique to electron transfer or electron capture dissociation. Proton transfer and electron transfer are competitive processes for any combination of anionic and cationic reactants. For reagent anions in reactions with protonated peptides, proton transfer is usually significantly more exothermic than electron transfer. If charge transfer occurs at relatively long distances, electron transfer should, therefore, be favored on kinetic grounds because the reactant and product channels cross at greater distances, provided conditions are favorable for electron transfer at the crossing point. The results are consistent with a model based on Landau-Zener theory that indicates both thermodynamic and geometric criteria apply for electron transfer involving polyatomic anions. Both the model and the data suggest that electron affinities associated with the anionic reagents greater than about 60-70 kcal/mol minimize the likelihood that electron transfer will be observed. Provided the electron affinity is not too high, the Franck-Condon factors associated with the anion and its corresponding neutral must not be too low. When one or the other of these criteria is not met, proton transfer tends to occur essentially exclusively. Experiments involving ion/ion attachment products also suggest that a significant barrier exists to the isomerization between chemical complexes that, if formed, lead to either proton transfer or electron transfer.
Figures


Similar articles
-
Where does the electron go? Electron distribution and reactivity of peptide cation radicals formed by electron transfer in the gas phase.J Am Chem Soc. 2008 Jul 9;130(27):8818-33. doi: 10.1021/ja8019005. J Am Chem Soc. 2008. PMID: 18597436
-
Effects of cation charge-site identity and position on electron-transfer dissociation of polypeptide cations.J Am Chem Soc. 2007 Oct 10;129(40):12232-43. doi: 10.1021/ja0736764. Epub 2007 Sep 19. J Am Chem Soc. 2007. PMID: 17880074
-
Electron transfer ion/ion reactions in a three-dimensional quadrupole ion trap: reactions of doubly and triply protonated peptides with SO2*-.Anal Chem. 2005 Mar 15;77(6):1831-9. doi: 10.1021/ac0483872. Anal Chem. 2005. PMID: 15762593 Free PMC article.
-
Ion cyclotron resonance spectroscopy. Cyclotron double resonance provides a new technique for the study of ion-molecule reaction mechanisms.Science. 1968 Jan 19;159(3812):263-73. doi: 10.1126/science.159.3812.263. Science. 1968. PMID: 4863791 Review.
-
Ion/ion chemistry of high-mass multiply charged ions.Mass Spectrom Rev. 1998 Nov-Dec;17(6):369-407. doi: 10.1002/(SICI)1098-2787(1998)17:6<369::AID-MAS1>3.0.CO;2-J. Mass Spectrom Rev. 1998. PMID: 10360331 Review.
Cited by
-
Alternately pulsed nanoelectrospray ionization/atmospheric pressure chemical ionization for ion/ion reactions in an electrodynamic ion trap.Anal Chem. 2006 May 1;78(9):3208-12. doi: 10.1021/ac052288m. Anal Chem. 2006. PMID: 16643016 Free PMC article.
-
Infrared photoactivation reduces peptide folding and hydrogen-atom migration following ETD tandem mass spectrometry.Angew Chem Int Ed Engl. 2009;48(45):8526-8. doi: 10.1002/anie.200903557. Angew Chem Int Ed Engl. 2009. PMID: 19795429 Free PMC article. No abstract available.
-
Covalent modification of gaseous peptide ions with N-hydroxysuccinimide ester reagent ions.J Am Chem Soc. 2010 Dec 29;132(51):18248-57. doi: 10.1021/ja107286p. Epub 2010 Dec 3. J Am Chem Soc. 2010. PMID: 21128662 Free PMC article.
-
Gas-phase ion/ion reactions of transition metal complex cations with multiply charged oligodeoxynucleotide anions.J Am Soc Mass Spectrom. 2008 Feb;19(2):281-93. doi: 10.1016/j.jasms.2007.10.017. Epub 2007 Nov 1. J Am Soc Mass Spectrom. 2008. PMID: 18083525
-
Fragmentation of alpha-radical cations of arginine-containing peptides.J Am Soc Mass Spectrom. 2010 Apr;21(4):511-21. doi: 10.1016/j.jasms.2009.12.021. Epub 2010 Jan 11. J Am Soc Mass Spectrom. 2010. PMID: 20138543
References
-
- Zubarev RA, Kelleher NL, McLafferty FW. J Am Chem Soc. 1998;120:3265–3266.
- Zubarev RA, Horn DM, Fridricksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BA, McLafferty FW. Anal Chem. 2000;72:563–573. - PubMed
- Zubarev RA. Mass Spectrom Rev. 2003;22:57–77. - PubMed
- Zubarev RA, Haselmann BB, Kjeldsen F, Jensen F. Eur J Mass Spectrom. 2002;8:337–349.
-
- Zubarev RA, Kruger NA, Fridriksson EK, Lewis MA, Horn DM, Carpenter BK, McLafferty FW. J Am Chem Soc. 1999;121:2857–2862.
- Kelleher NL, Zubarev RA, Bush K, Furie B, Furie BC, McLafferty FW, Walsh CT. Anal Chem. 1999;71:4250–4253. - PubMed
- Mirgorodskaya E, Roepstorff P, Zubarev RA. Anal Chem. 1999;71:4431–4436. - PubMed
- Horn DM, Breuker K, Frank AJ, McLafferty FW. J Am Chem Soc. 2001;123:9792–9799. - PubMed
- Håkansson K, Cooper HJ, Emmett MR, Costello CE, Marshall AG, Nilsson CL. Anal Chem. 2001;73:4530–4536. - PubMed
- Sze SK, Ge Y, Oh H, McLafferty FW. Proc Natl Acad Sci USA. 2002;99:1774–1779. - PMC - PubMed
- Shi SDH, Hemling ME, Carr SA, Horn DM, Lindh I, McLafferty FW. Anal Chem. 2001;73:19–22. - PubMed
- Håkansson K, Chalmers MJ, Quinn JP, McFarland MA, Hendrickson CL, Marshall AG. Anal Chem. 2003;75:3256–3262. - PubMed
-
- Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc Natl Acad Sci USA. 2004;101:9528–9533. - PMC - PubMed
- Coon JJ, Syka JEP, Schwartz JC, Shabanowitz J, Hunt DF. Int J Mass Spectrom. 2004;236:33–42.
- Pitteri SJ, Chrisman PA, Hogan JM, McLuckey SA. Anal Chem. 2005;77:1831–1839. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

