Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation
- PMID: 16144801
- PMCID: PMC1895247
- DOI: 10.1182/blood-2005-06-2217
Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation
Abstract
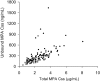
The immunosuppressive drug mycophenolate mofetil (MMF) is used after nonmyeloablative hematopoietic cell transplantation (HCT); however, limited pharmacodynamic data are available. We evaluated plasma concentrations of mycophenolic acid (MPA), the active metabolite of MMF, and outcomes in 85 patients with hematologic malignancies conditioned with fludarabine and 2 Gy total body irradiation followed by HLA-matched unrelated-donor HCT and postgrafting cyclosporine and MMF. The first 38 patients received MMF 15 mg/kg twice daily; the next 47 patients received MMF 3 times daily. MPA pharmacokinetics were determined on days 7 and 21. Comparing the twice-daily and 3-times-daily MMF groups, the mean total MPA concentration steady state (Css) was 1.9 and 3.1 microg/mL; the unbound Css was 18 and 36 ng/mL, respectively (P < .001). Sixteen patients with a total MPA Css less than 3 microg/mL had low (< 50%) donor T-cell chimerism (P = .03), and 6 patients with MPA Css less than 2.5 microg/mL had graft rejection. An elevated unbound Css was associated with cytomegalovirus reactivation (P = .03). There were no significant associations between MPA pharmacokinetics and acute graft-versus-host disease (GVHD) or relapse. We conclude that increased MPA Css's predicted higher degrees of donor T-cell chimerism after unrelated donor nonmyeloablative HCT and suggest that targeting MPA Css's greater than 2.5 microg/mL could prevent graft rejection.
Figures



References
-
- McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97: 3390-3400. - PubMed
-
- Champlin R, Khouri I, Kornblau S, Molldrem J, Giralt S. Reinventing bone marrow transplantation: reducing toxicity using nonmyeloablative, preparative regimens and induction of graft-versus-malignancy (Review). Curr Opin Oncol. 1999;11: 87-95. - PubMed
-
- Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94: 3234-3241. - PubMed
-
- Georges GE, Maris M, Sandmaier BM, et al. Related and unrelated nonmyeloablative hematopoietic stem cell transplantation for malignant diseases. Int J Hematol. 2002;76(suppl 1): 184-189. - PubMed
-
- Barrett AJ. Conditioning regimens for allogeneic stem cell transplants (Review). Curr Opin Hematol. 2000;7: 339-342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

