LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages
- PMID: 16144905
- PMCID: PMC2171430
- DOI: 10.1083/jcb.200505166
LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages
Abstract
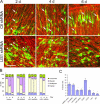
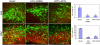
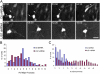
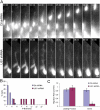
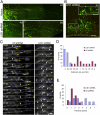
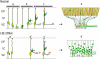
Mutations in the human LIS1 gene cause the smooth brain disease classical lissencephaly. To understand the underlying mechanisms, we conducted in situ live cell imaging analysis of LIS1 function throughout the entire radial migration pathway. In utero electroporation of LIS1 small interference RNA and short hairpin dominant negative LIS1 and dynactin cDNAs caused a dramatic accumulation of multipolar progenitor cells within the subventricular zone of embryonic rat brains. This effect resulted from a complete failure in progression from the multipolar to the migratory bipolar state, as revealed by time-lapse analysis of brain slices. Surprisingly, interkinetic nuclear oscillations in the radial glial progenitors were also abolished, as were cell divisions at the ventricular surface. Those few bipolar cells that reached the intermediate zone also exhibited a complete block in somal translocation, although, remarkably, process extension persisted. Finally, axonal growth also ceased. These results identify multiple distinct and novel roles for LIS1 in nucleokinesis and process dynamics and suggest that nuclear position controls neural progenitor cell division.
Figures


Comment in
-
LIS-less neurons don't even make it to the starting gate.J Cell Biol. 2005 Sep 12;170(6):867-71. doi: 10.1083/jcb.200506140. J Cell Biol. 2005. PMID: 16157697 Free PMC article. Review.
References
-
- Ahmad, F.J., J. Hughey, T. Wittmann, A. Hyman, M. Greaser, and P.W. Baas. 2000. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat. Cell Biol. 2:276–280. - PubMed
-
- Dobyns, W.B., and C.L. Truwit. 1995. Lissencephaly and other malformations of cortical development: 1995 update. Neuropediatrics. 26:132–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

