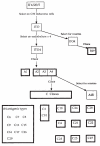
Rapid switching to multiple antigenic and adhesive phenotypes in malaria
- PMID: 1614515
- PMCID: PMC3731710
- DOI: 10.1038/357689a0
Rapid switching to multiple antigenic and adhesive phenotypes in malaria
Abstract
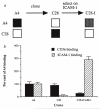
Adhesion of parasitized erythrocytes to post-capillary venular endothelium or uninfected red cells is strongly implicated in the pathogenesis of severe Plasmodium falciparum malaria. Neoantigens at the infected red-cell surface adhere to a variety of host receptors, demonstrate serological diversity in field isolates and may also be a target of the host-protective immune response. Here we use sequential cloning of P. falciparum by micromanipulation to investigate the ability of a parasite to switch antigenic and cytoadherence phenotypes. Our data show that antigens at the parasitized cell surface undergo clonal variation in vitro in the absence of immune pressure at the rate of 2% per generation with concomitant modulations of the adhesive phenotype. A clone has the potential to switch at high frequency to a variety of antigenic and adhesive phenotypes, including a new type of cytoadherence behaviour, 'auto-agglutination' of infected erythrocytes. This rapid appearance of antigenic and functional heterogeneity has important implications for pathogenesis and acquired immunity.
Figures
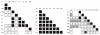
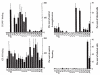
Comment in
-
Malaria. Asexual deviants take over.Nature. 1992 Jun 25;357(6380):647-8. doi: 10.1038/357647a0. Nature. 1992. PMID: 1614513 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

