Dual phase and rate coding in hippocampal place cells: theoretical significance and relationship to entorhinal grid cells
- PMID: 16145693
- PMCID: PMC2677681
- DOI: 10.1002/hipo.20115
Dual phase and rate coding in hippocampal place cells: theoretical significance and relationship to entorhinal grid cells
Abstract
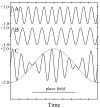
We review the ideas and data behind the hypothesis that hippocampal pyramidal cells encode information by their phase of firing relative to the theta rhythm of the EEG. Particular focus is given to the further hypothesis that variations in firing rate can encode information independently from that encoded by firing phase. We discuss possible explanation of the phase-precession effect in terms of interference between two independent oscillatory influences on the pyramidal cell membrane potential, and the extent to which firing phase reflects internal dynamics or external (environmental) variables. Finally, we propose a model of the firing of the recently discovered "grid cells" in entorhinal cortex as part of a path-integration system, in combination with place cells and head-direction cells.
Copyright 2005 Wiley-Liss, Inc.
Figures





References
-
- Alonso A, Llinas RR. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:175–177. - PubMed
-
- Bullock TH, Buzsaki G, McClune MC. Coherence of compound field potentials reveals discontinuities in the CA1-subiculum of the hippocampus in freely-moving rats. Neuroscience. 1990;38:609–619. - PubMed
-
- Burgess N, Recce M, O’Keefe J. A model of hippocampal function. Neural Networks. 1994;7:1065–1081.
-
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

