Stringent doxycycline-dependent control of gene activities using an episomal one-vector system
- PMID: 16147984
- PMCID: PMC1201338
- DOI: 10.1093/nar/gni137
Stringent doxycycline-dependent control of gene activities using an episomal one-vector system
Abstract
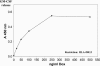
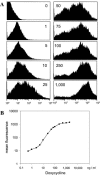
Conditional expression systems are of pivotal importance for the dissection of complex biological phenomena. Here, we describe a novel EBV-derived episomally replicating plasmid (pRTS-1) that carries all the elements for conditional expression of a gene of interest via Tet regulation. The vector is characterized by (i) low background activity, (ii) high inducibility in the presence of doxycycline (Dox) and (iii) graded response to increasing concentrations of the inducer. The chicken beta actin promoter and an element of the murine immunoglobin heavy chain intron enhancer drive constitutive expression of a bicistronic expression cassette that encodes the highly Dox-sensitive reverse tetracycline controlled transactivator rtTA2(S)-M2 and a Tet repressor-KRAB fusion protein (tTS(KRAB)) (silencer) placed downstream of an internal ribosomal entry site. The gene of interest is expressed from the bidirectional promoter P(tet)bi-1 that allows simultaneous expression of two genes, of which one may be used as surrogate marker for the expression of the gene of interest. Tight down regulation is achieved through binding of the silencer tTS(KRAB) to P(tet)bi-1 in the absence of Dox. Addition of Dox releases repression and via binding of rtTA2(S)-M2 activates P(tet)bi-1.
Figures





References
-
- Hillen W., Berens C. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 1994;48:345–369. - PubMed
-
- Gossen M., Bonin A.L., Bujard H. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem. Sci. 1993;18:471–475. - PubMed
-
- Berger S., Bujard H. Novel mouse models in biomedical research: the power of dissecting pathways by quantitative control of gene activities. In: Dathe S., editor. Handbook of Experimental Pharmacology. Heidelberg: Springer Verlag; 2003.
-
- Gossen M., Freundlieb S., Bender G., Muller G., Hillen W., Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

