Monoclonal immunoglobulin G1 directed against Aspergillus fumigatus cell wall glycoprotein protects against experimental murine aspergillosis
- PMID: 16148172
- PMCID: PMC1235786
- DOI: 10.1128/CDLI.12.9.1063-1068.2005
Monoclonal immunoglobulin G1 directed against Aspergillus fumigatus cell wall glycoprotein protects against experimental murine aspergillosis
Abstract
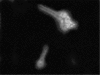
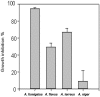
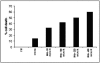
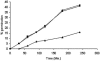
Most of the biological functions related to pathogenicity and virulence reside in the fungal cell wall, which, being the outermost part of the cell, mediates the host-fungus interplay. For these reasons much effort has focused on the discovery of useful inhibitors of cell wall glucan, chitin, and mannoprotein biosynthesis. In the absence of a wide-spectrum, safe, and potent antifungal agent, a new strategy for antifungal therapy is directed towards the development of monoclonal antibodies (MAbs). In the present study the MAb A9 (immunoglobulin G1 [IgG1]) was identified from hybridomas raised in BALB/c mice immunized with cell wall antigen of Aspergillus fumigatus. The immunoreactive epitopes for this IgG1 MAb appeared to be associated with a peptide moiety, and indirect immunofluorescence microscopy revealed its binding to the cell wall surface of hyphae as well as with swollen conidia. MAb A9 inhibited hyphal development as observed by MTT [3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (25.76%), reduced the duration of spore germination, and exerted an in vitro cidal effect against Aspergillus fumigatus. The in vivo protective efficacy of MAb A9 was also evaluated in a murine model of invasive aspergillosis, where a reduction in CFU (>4 log(10) units) was observed in kidney tissue of BALB/c mice challenged with A. fumigatus (2 x 10(5) CFU/ml) and where enhanced mean survival times (19.5 days) compared to the control (7.1 days) and an irrelevant MAb (6.1 days) were also observed.
Figures





References
-
- Breedveld, F. C. 2000. Therapeutic monoclonal antibodies. Lancet 355:735-740. - PubMed
-
- Casadevall, A., A. Cassone, F. Bistoni, J. F., Cutler, W. Magliani, J. W. Murphy, L. Polonelli, and L. Romani. 1998. Antibody and/or cell-mediated immunity, protective mechanisms in fungal disease: an ongoing dilemma or an unnecessary dispute? Med. Mycol. 36:95-105. - PubMed
-
- Cassone, A., A. Torosantucci, M. Boccanera, G. Pellengrini, C. Palma, and G. Malavasi. 1988. Production and characterization of a monoclonal antibody to a cell surface, glucomannoprotein constituent of Candida albicans and other pathogenic Candida species. J. Med. Microbiol. 27:233-238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

