Cdh1/Hct1-APC is essential for the survival of postmitotic neurons
- PMID: 16148219
- PMCID: PMC6725543
- DOI: 10.1523/JNEUROSCI.1143-05.2005
Cdh1/Hct1-APC is essential for the survival of postmitotic neurons
Abstract
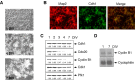
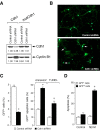
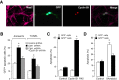
Cell division at the end of mitosis and G1 is controlled by Cdh1/Hct1, an activator of the E3-ubiquitin ligase anaphase-promoting complex (APC) that promotes the ubiquitylation and degradation of mitotic cyclins and other substrates. Cdh1-APC is active in postmitotic neurons, where it regulates axonal growth and patterning in the developing brain. However, it remains unknown whether Cdh1-APC is involved in preventing cell-cycle progression in terminally differentiated neurons. To address this issue, we used the small hairpin RNA strategy to deplete Cdh1 in postmitotic neurons. We observed that Cdh1 silencing rapidly triggered apoptotic neuronal death. To investigate the underlying mechanism, we focused on cyclin B1, a major Cdh1-APC substrate. Our results demonstrate that Cdh1 is required to prevent the accumulation of cyclin B1 in terminally differentiated neurons. Moreover, by keeping cyclin B1 low, Cdh1 prevented these neurons from entering an aberrant S phase that led to apoptotic cell death. These results provide an explanation for the mechanism of cyclin B1 reactivation that occurs in the brain of patients suffering from neurodegenerative diseases, such as Alzheimer's disease.
Figures



References
-
- Almeida A, Moncada S, Bolaños JP (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6: 45–51. - PubMed
-
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M (2004) Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428: 190–193. - PubMed
-
- Becker EBE, Bonni A (2004) Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol 72: 1–25. - PubMed
-
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
